Separating components with different physical properties: Application of different filtration techniques

One of the most important processes at the moment of working with mixtures, are the techniques the separation of its different components; with simple treatments as the evaporation this can be achieved, however the technique that must be applied will depend on the properties that present each one of the compounds that are desired to separate. The sublimation, precipitation, crystallization and filtration will be applied depending on the result to be obtained, even so there are more sophisticated techniques such as chromatography, which is much more complex than the previous ones, but remarkably efficient. The importance of these processes lies in the quantitative study of the components present and the proportion between them. As a problem sample, one containing benzoic acid, sand and lead (II) nitrate is considered, in order to explain in an experimental and methodological way the aforementioned processes. By means of various filtration techniques (gravity filtration with simple folding/folding of the filter paper and suction filtration), it was possible to separate each of these three components. The sand was discarded, while lead (II) nitrate was obtained in solution and reacted with potassium iodide (KI) to obtain solid lead (II) iodide (PbI2); on the other hand benzoic acid was also obtained in solid state after being purified with activated carbon. Each of these separations represents an essential technique in many fields of classical chemical analysis.
Theoretical Basis
A mixture can be defined as a material system formed by two or more components joined, but not chemically combined, which can be separated by several methods, of which this post stands out, filtration. When filtering a mixture is of great importance to know the different techniques that can be handled, since some are more convenient than others depending on the characteristics of the mixture problem.

a) Gravity filtration occurs when a liquid passes through a filter due to its own weight, it is done using a funnel and filter paper. By folding the filter paper simply (in the form of a cone) a system is achieved that allows solids to be retained efficiently, but relatively slowly, in this case a funnel with a stem is used.
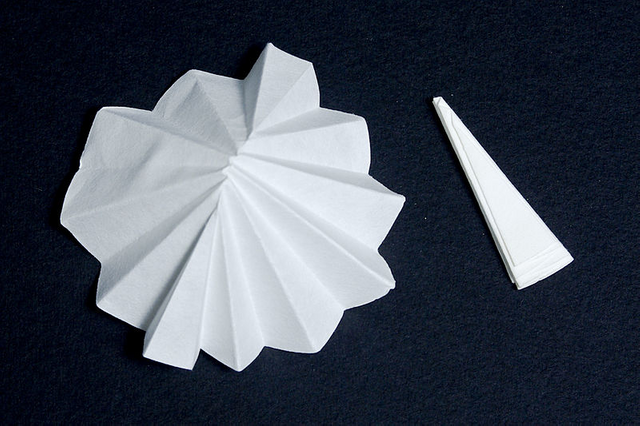
b) One way to speed up the passage of liquid through the filter paper is to increase the exposure area between the two (the mixture and the paper), this is achieved by performing a folding in the form of folds to the paper, this technique is known as gravity filtration with folded paper, quite useful when filtering hot or cold liquids.

c) One technique that focuses on solids recovery rather than filtration is suction filtration using a Buchner funnel and kitasate, along with a suction system.
Mixtures can be classified into heterogeneous mixtures, when their components can be recognized with the naked eye in a simple way and they are homogeneous when their components cannot be distinguished so easily (a single phase is formed).
In some cases it is necessary to use activated carbon to purify solutions, which are highly crystalline carbonaceous adsorbents and highly developed internal porosity. This type of particle performs an adsorption process (the process of attracting the molecules or ions of one substance to the surface of another, the most common type being the adhesion of liquids and gases to the surface of solids), thus retaining the contaminating particles of a solution.


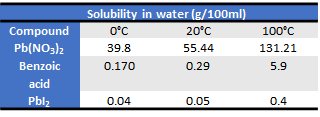
In certain cases, the recrystallization process can facilitate the separation of a compound if its precipitation (or formation of crystalline structures) contemplates larger solids. Below are some solubility values of the compounds of interest for this post.
And reactions of interest to those compounds:
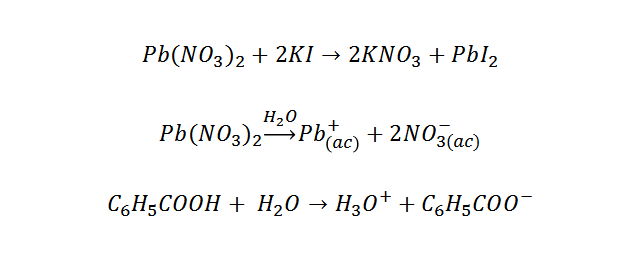
The classical treatment for a simple sample is shown below in an experimental way:
1. Obtaining Pb(NO3)2 in solution.
Procedure:
The mixture of sand, benzoic acid and lead nitrate (II) was weighed in a beaker (previously weighed) and by difference of mass 4 grams of it were obtained, a granular scale with a resolution of 0.05 g was used. Then 30 ml of distilled water was added and the material was agitated to dissolve and then placed in an ice bath for approximately 20 minutes. Finally, a gravity filtration system was prepared using folded paper and the sample was filtered completely and quickly to prevent it from getting hot.
Results:
Initially the given sample contained notably sand, however, it was not simple to distinguish lead nitrate (II) and benzoic acid as both were white in colour.
After adding the 30 ml of water it was agitated to be able to dissolve all the lead (II) nitrate, which is quite soluble in water and will hardly remain solid during filtration. Cooling is done in order to reduce the solubility of benzoic acid, which at 0° C is only 0.170/100ml, in a solution of 30 ml the amount of solubilized benzoic acid would be 0.051 g, it is precisely this amount which cannot be filtered (it is dissolved). After the solution had been in an ice bath for 20 minutes, a greater amount of white solid (benzoic acid) was observed in the solution. The solution was quickly filtered using the folded paper technique (see image of this type of paper), to ensure rapid filtration and thus avoid a heating that could increase the solubility of benzoic acid, increasing its loss. Finally, a solution of lead nitrate (II) and a filtrate containing benzoic acid and sand was obtained.
2. Precipitation and separation of Pb(II) as PbI2.
Procedure:
To the filtrate obtained in the previous experiment was added 15 ml of potassium iodide (KI) 0.1M, then at the end of the reaction was diluted with 40 ml d distilled water, heated to dissolve all the solute and left to cool to room temperature.
On the other hand, the filter paper used in a watch glass was weighed. Then the system was prepared to filter with a paper with simple folding (not being working with hot or cold solutions it is not necessary to carry out the filtering quickly). Once the solution had cooled, the filtration was carried out, making sure that all the solid (lead iodide (II) remained in the filter paper, and the necessary washes were made for this.
Once the filter paper had been removed with the solid, it was placed in a stove to dry the residue. Finally, the filter paper was weighed again on the watch glass and the mass difference was used to determine the mass of the precipitate obtained.
Results:
It was of great importance to measure the initial weight of the filter paper together with the watch glass, to finally determine the amount of lead iodide (II) (PbI2) formed (after being dried in the stove) and with this figure to stoichiometrically estimate the initial amount of lead nitrate (II) (Pb(NO3)2) contained in the solution obtained in the previous experiment.
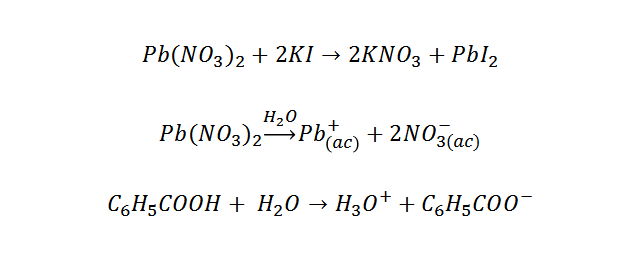
A recrystallization of PbI2 was needed, since the solid initially formed was very small and could not be filtered (it obstructed the pores of the filter paper), after heating, dissolving and recrystallizing a relatively larger solid was formed that facilitated the filtration process.

It should be noted that when the potassium nitrate solution (KNO3) was filtered a small amount of dissolved lead iodide remained, in this case the solution measured was 70 ml, therefore, 0.035 g of PbI2 was solubilized at room temperature (amount that could not be retained in the filter paper). By adding the filtered amount with the unfiltered amount we obtain approximately 0.54 ± 0.07 grams of lead (II) iodide, with which we can estimate the amount of lead nitrate that was initially present.
Lead (II) nitrate is in a 1:1 ratio with lead (II) iodide, both molecules contain only one lead atom. By calculating the moles of PbI2, we will obtain the moles of lead (II) nitrate, by stoichiometric ratio, this amount of moles corresponds to the moles of lead (II) nitrate, then calculating the grams:
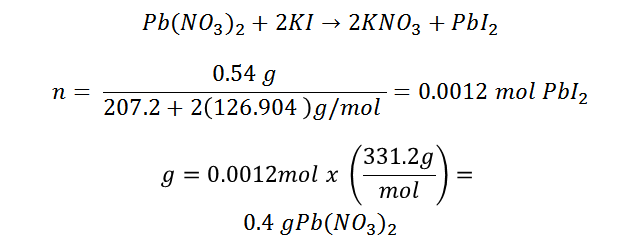
In this way the amount of lead (II) nitrate present in the initial sample can be estimated.
3. Obtaining benzoic acid in solution.
Procedure:
The residue contained in the filter paper from experience 1 was heated in a 100 ml beaker with 50 ml of distilled water. A filter system with folded paper was prepared in order to be able to quickly filter the hot solution. Once the start of boiling was observed, the liquid was quickly filtered, the residue (sand) was discarded and the filtrate (benzoic acid solution) was preserved.
Results:
In this experience it was convenient to heat the residue formed by sand and benzoic acid at boiling water temperature, to increase the solubility of benzoic acid and be able to obtain a sand free solution by filtration with filter paper folded by folds. At 100° C the solubility of benzoic acid is 5.9 g/100ml as there was only 50ml of water so the solubility was 2.95 grams.
Observations: When the mixture was heated, the benzoic acid (white solid) was dissolved, and after filtration a brownish residue (sand) was obtained, which was discarded.
As the resulting solution was not completely colorless, it was then purified.
4. Purification of benzoic acid by activated carbon .
Procedure:
To the beaker with the filtering of experience 3, 1 gram of activated carbon was added, then it was heated to boiling and the hot liquid was filtered with the technique of filtering with folded paper.
Results:
The solution became completely black after adding 1 gram of activated carbon, when heated many bubbles were generated, after filtering it turned out to be colorless (with some particles of activated carbon).
It was also observed the capacity of the activated carbon to adsorb the particles that contaminated the solution of benzoic acid, since at that moment the solution was still pink product of the dye mentioned above, after filtering the dye ceased to be in the solution and remained in the filter paper attached to the activated carbon.

5. Obtaining benzoic acid.
Procedure:
The purified benzoic acid solution obtained from the previous experience was heated to boiling point, thus achieving the evaporation of half of its volume. It was then cooled and placed in an ice bath for approximately 20 minutes.
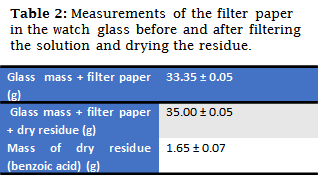
A suction filtration system was prepared, and the mass of the filter paper (which would be used) was determined along with the watch glass. Filtration was then performed to the maximum extraction of any liquid that might be trapped in the solid benzoic acid. The filter paper was then moved in a watch glass to a stove to dry any liquid that remained in the filter paper. Finally the filter paper was weighed again on the watch glass and by mass difference the amount in grams of benzoic acid that was filtered was determined.
Results:
In this experience the mass of benzoic acid that had been purified was finally obtained.
When the solution of the benzoic acid was cooled, the precipitate of the same was formed, product of the decrease of its solubility in the new temperature, when filtering this solid was deposited in the filter paper, however again part of the benzoic acid was not retained thanks to the solubility of this compound at 0° C, which is 0.170 g/100ml and having 25 ml the solubilized grams were 0.043 g. This small amount must be taken into account for the final quantitative analysis.

Once the residue dried, small black traces were observed, corresponding to the activated carbon. It can be said that the filtrate was not 100% effective since these small traces disturb the measurement of the real mass of the acid, and therefore must be taken as an error.
Based on the Results obtained in each of the previous experiences, the real composition of the sample could be calculated taking into account the proportions of the components found in it. It should be noted that, in all the measurements taken, systematic errors associated with the instruments (balance, graduated cylinder) could have been present, as well as random errors that could not be detected. One of the errors that must be taken into account are the traces of activated carbon found in the solid of benzoic acid at the end of its drying, therefore, the mass of the same will be slightly higher than the real value.
In the case of benzoic acid there was a loss of solids in experience 1 of 0.051 grams (part that was kept in solution); then in experience 3 and 4 one could think of some type of loss by solubility, however if the final result is taken into account, the final sample had a mass approximately (without taking into account some errors) 1.65 grams; then as in experience 3 and 4 the solubility was heated to 100°C in both cases, taking into account the volumes associated to each experiment was much greater than 1.65, so in these solutions, the benzoic acid did not saturate the solvent. It is important to emphasize that this analysis could only be done with the final result, if the mass obtained finally exceeded the solubility of the compound for the cases exposed above, if it could be a loss by precipitation.
In experience 5, 0.043 g of benzoic acid was lost in the volume of its solution, and that small amount was kept in solution.

Once the losses have been taken into account, the presence of traces of activated carbon must be highlighted, which necessarily increase the mass of the sample, consequently, the real value must be:
Real value < 1.56 ± 0.07
By studying the amount of silver nitrate it was found that part of the lead iodide (II) could not be solubilized (0.035 g). This amount was taken into account for the calculation of the initial grams of lead (II) nitrate, which were approximately 4g, (the lost part does not represent much variation in the final result).
For difference of masses, one can determine the grams of sand which are approximately 2.04g.

Once the sample was processed, it was possible to observe how the understanding of the functioning of the filtration techniques significantly optimized the data obtained, since some solutions (cold or hot) merited a fast and precise filtration that was only achieved with the folded paper technique.
The presence of errors cannot be ruled out and as a measure to reduce their impact on the real value, it would be possible to compare the repetitions performed and calculate their standard deviation in order to achieve a more accurate value. Care should be taken with the filtering and its appearance, since the observation of the same allows us to conclude if the technique has been applied successfully.
Activated carbon proved to be a good filter that was able to adsorb most of the traces that contaminated the benzoic acid sample.
Once again filtration techniques are fundamental for chemical analysis, but knowledge about the solubility and other physical-chemical properties of the compounds to be treated is also important, since it is precisely this that will determine the technique that should or should be used, definitely indispensable for quantitative chemistry.
We are still waiting for the great developments that the future will bring us, you decide whether to be a spectator or a director. Every day we can learn something new.
Thank you for reading.
References:
All the images and dividers of my authorship were edited and processed using the software PowerPoint 2016.
- Theodore Brown, Eugene LeMay, Bruce Bursten, Julia Burdge, (2004), Chemistry. The central science. (9th Edition). Pearson Education, S.A., Chapter 04.
Douglas Skoog, Donald West and James Holler (2006) Fundamentals of Chemistry Analytical (4th Edition) Editorial Reverté.
CRC HandBook of Chemistry and Physics, 86th Edition, CRC Press 2005
Arthur I. Vogel et al. (1989) Textbook of Quantitative Chemical Analysis, 5th edition, Longman Scientific & Technical.

Posted from my blog with SteemPress : http://aleestra.vornix.blog/separating-components-with-different-physical-properties-application-of-different-filtration-techniques/
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @curie.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Aleestra from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
Congratulations @aleestra! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Congratulations @aleestra! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Congratulations @aleestra! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard: