My project on bio fuel from lignocellulose materials(Coconut shell and groundnut shell)
"
Cellulosic ethanol is a type of bio fuel produced from lignocelluloses, a structural material that comprises much of the mass of plant. Lignocellulose is composed of majorly cellulose, hemicelluloses and lignin. Cellulosic ethanol can be obtained from Corn Stover, pernicum vegatum (switch grass), coconut shell, groundnut shell, miscanthus grass species, woodchip and the by product of lawn and tree maintenance. Ethanol obtained from lignocellulosic material has their advantage and abundance raw material.
One of the major benefits of cellulosic ethanol is that it reduces greenhouse gas emission (GHG) by 85% over reformulated gasoline.
There is an endless search for renewable sources of fuels due to the non-renewability of fossil fuels. The continuous increase in population of both the developed and developing countries with its increase in energy consumption and the non-renewability of the energy source has prompted the search for an alternative energy source.
Ethanol can also be produced from corn, cassava, sugarcane and so on, but these sources could pose treat to feeding. Thus, the use of residual lignocellulosic material is more economical and has reduced both the soil erosion and land clearing associated with corn ethanol. Moreover, large residual lignocellulosic material are available domestically (Fargione et al., 2008).
Cellulosic ethanol is a bio fuel which is an alternative form of energy. It is produced from lignocellulosic materials (coconut shell, ground nut shell, and so on.) through various processes such as dilute acid hydrolysis, concentrated acid hydrolysis and enzymatic hydrolysis. It is used as anti-oxidant, bio fuel and soil binder.
**CHEMICAL COMPOSITION OF LIGNOCELLULOSE**
- HEMICELLULOSE
A hemicellulose (which is also referred to as polyose) is one the several heteropolymer (matrix polysaccharide), such as arabiloxylan, which is present along with cellulose in all plant cell walls. While cellulose is a crystalline, strong and resistance to hydrolysis, hemicelluloses has a random amorphous structure with little strength. It is easily hydrolysed by dilute acid or base as well as myriad (Wikipedia, 2016). The major composition of hemicellulose is xylan, arabinoxylan, glucuronoxylen, glucomannan and xyloglucan. All the aforementioned polysaccharides contain several sugar monomers. At times, hemicelluloses may contain small amount of L-sugar and mostly D-pentose.
- LIGNIN
Lignin is a complex organic polymer that forms important structural material in the support tissue of vascular plants and some algae. Lignin’s are important in the formation of cell walls, especially wood and bark, because they lend rigidity and do no rot easily (Wikipedia, 2016). Chemically, lignin is a cross-linked phenolic polymer. The composition of lignin varies from species to species.
It is heterogeneous and lack defined primary structure. This makes it unique and hence it's property as support mechanism through strengthening of the wood (xylem cells in trees). Lignin is a giant molecule with molecular weight in excess of 10,000 units and relatively hydrophobic and aromatic in nature. Lignin is made up of repetitive structures occurring in haphazard manner that is, p-coumaryl, coniferyl alcohol and sinapyl alcohol. These monolignols are incorporated into the lignin like the phenyl propanoids. P-hydroxylphenyl (H), guaiacyl (G) and syringyl (S) respectively.
- CELLULOSE
This is an organic compound with formula (C6H10O5)n, a polysaccharide made up of linear chain of several hundred to many thousands of beta (1-4) linked D-glucose mostly combined with lignin and other polysaccharides in the cell wall of a woody plant. Cellulose is a crystalline, strong and hydrolysis resistant substance; it constitutes the most abundant renewable polymer material available today in the world. It is mainly used to produce paperboard and paper.
Cellulose has no taste, is odourless, hydrophilic with contact angle of 20-300 (Bishop et al., 2007) and biodegradable. Cellulose is derived from D-glucose units, which condense through telo:
h beta(1-4) glycosidic bonds. Cellulose is a straight chain polymer, the molecule adopts an extended and rather stiff rod-like conformation aided by the equatorial conformation of the glucose residue. As a polydispersed linear homopolymer, it consist of regio and enantio selectively-1,4- glycosidic linked d-glucopyranose units. As shown by HNMR spectroscopy, the -d-glucopyranose adopts the 4Cl chain conformation. It contains free O-H-group at the C-2, C-3 and C-6 atoms.
STATEMENT OF PROBLEM
The high dependency on fossil fuel as source of energy has increased the rate of deforestation and the non-renewability of fossil fuel combine with the adverse effect on the environment has threatened the lives of people and the ecosystem has prompted a need for alternative energy source which is environmentally benign. Lignocellulosic material such as coconut shell and groundnut shell are termed as waste and often improperly discarded into the environment, this has constituted waste disposal problem to the environment.
The practice of mechanized farming has led to extensive discharge of agricultural wastes that have had negative effects on the environment. The utilization of such wastes has been a source of concern to many researchers (Amosun, 2000). Therefore, this work was designed to look into the possibility of converting some of such by-products into industrial chemicals of economic importance.
JUSTIFICATION
Studies have shown that cellulosic ethanol which is a bio-fuel is a good alternative source of energy due to its renewability, non-toxicity and environmentally friendly. The waste generated from coconut and groundnut shells in Minna anquanbiri are discarded indiscriminately, the improper disposal of this lignocellulosic material posses environmental problem. Thus, adequate recycling of these materials (coconut and groundnut shells) for the production of cellulosic ethanol will poses a lot of economic importance.
AIM AND OBJECTIVE
Aim
The aim of this study is to produce cellulosic ethanol (a renewable energy source which could be used as an alternative to fossil fuel) from lignocellulosic material such as coconut shell and groundnut shell.
Objectives;
Other specific objectives include;
Produce cellulosic ethanol from coconut shell and groundnut shell which are refer to as lignocellulosic material.
Characterise the cellulosic ethanol produced from lignocellulosic material (coconut shell and groundnut shell).
Compare the yield of cellulosic ethanol produced from both materials (groundnut shell and coconut shell.
Determine the chemical component of groundnut and coconut shell.
SCOPE OF STUDY
in this study the conversion of cellulose from energy crop into biofuels such as cellulosic ethanol shall be investigated as an alternative fuel source.
MATERIALS AND METHODS
Materials
Coconut and groundnutshell, Yeast,
3.1.1 Chemicals used
Sulphuric acid, Sodium hydroxide, Distilled water
3.1.2 Apparatus used
Electric oven, Volumetric flask, Conical flask, Digital weighing balance, Filter cloth, Heating mantle, Blender, Claisen condenser Thermometer Foil paper Funnel.
3.2 METHODS
3.2.1 Sample Collection
Coconuts and groundnut shells were collected in polythene at U.rimi kaduna State, Nigeria and transported to the laboratory. The collected samples were washed to remove dirt. Thereafter it was broken into smaller sizes and then blended into pulp.
3.2.2 Pretreatment
200g of each sample (coconut and groundnut shell) washed with tap water by pouring the samples into 500cm3 of the tap and the mixture was parboiled for 20 minutes at a temperature of 50°C in order to remove water any gross dirt and contamination then it was left to cool for 15 minutes and the decanted and poured away. Another 400cm3 of tap water was poured and stirred to ensure complete washing then the sample ware gradually collected from the top till there was only a small fraction left in the dirty water and this was poured away. The collected samples were then dried for an hour using the laboratory oven at temperature of 80oC till it was about 10% moisture content. After which the sample was poured into a wooden mortar and was pounded to the size of 5mm.
The process was repeated for another 100g of the two samples but were pounded to the size of 0.85mm.
The processes where repeated all over again for another long of the sawdust but was reduced to the size of 0.2mm.
3.2.3 Chemical characterization
3.2.3.1 Hemicellucloses
Procedure
2 g of vacuum-oven dried holocelluose was measured and place into a 250 ml glass beaker.10 ml of 17.5% NaOH solution was added to the holocellulose in a 250-ml beaker, cover with a watch glass, and maintain at 200C in a water bath. the holocellulose was manipulated lightly with a glass rod with a flat end so that the entire specimen becomes soaked with the NaOH solution. After the addition of the first portion of 17.5% NaOH solution to the specimen, at five minute intervals, 5 ml more of the NaOH solution was added and thoroughly stir the mixture with the glass rod. this procedure was continued until the NaOH is consumed. the mighty was allowed to stand at 200C for 30 min. making the total time for NaOH treatment 45 min. 33 ml of distilled water was added at 200C to the mixture. Thoroughly mixture of the beaker and allow to stand at 200C for 1 hour before filtering. Filter the cellulose with the aid of suction into the tarred, alkali-resistant Alundu or fritted glass crucible of medium porosity. the entire holocellulose residue was transferred to the crucible, and wash with 100 ml of 3% NaOH solution at 200C. After the NaOH wash solution has passed through the residue in the crucible, the washing at 200C with distilled Hater was continued, making certain that all particles have been transferred from the 250-ml beaker to the crucible. Washing the sample in the crucible is facilitated by releasing the suctions filling the crucible to within 6 mm of the top with water. Repeat this step twice. 15 ml o f 10% acetic acid at room temperature was poured into the crucible, drawing the acid into the cellulose by suction but, while the cellulose is still covered with acid, the suction was released. Then Subject the cellulose to the acid treatment for 3 min. from the time the suction is released then apply suction to draw off the acetic acid. Without releasing the suction, then fill the crucible almost to the top with distilled water at 200C and allow to it drain completely. Repeat the washing until the cellulose residue is free of acid as indicated by litmus Paper give the cellulose a final washing by drawing, by suctions, an additional 250 ml of distilled water through the cellulose in the crucible. Dry the crucible on the bottom and sides with a cloth and place it overnight in an oven dry at l050C. Cool the crucible and weighing bottle in a desiccator for 1 hour before weighing.
3.2.3.2 CELLULOSE
Procedure
To 2.5 g of sample, 80 ml of hot distilled water was added, 0.5 ml acetic acid, and sodium chlorite in 250 ml Erlenmeyer flask. An optional 25 ml Erlenmeyer flask is inverted in the neck of the reaction flask. The mixture is heated in a water bath at 700C. After 60 minutes, 0.5 ml of acetic acid and 1 g of sodium chlorite are added with shaking. The delignification process degrades. Continue reaction will remove more lignin but hemicelluloses will also be lost (Rowel, 1980). Addition of 0.5 ml of acetic acid and 1 g of sodium chlorite is repeated until the sample is completely separated from lignin which usually takes 6 to 8 hour of cholriting. At the end of 24 hours of reaction, the sample was cooled and filter the hollocelulose on filter paper using a Buchner Funnel until the yellow color (the color of hollocelulose is white) and the odor o f chlorine dioxide is removed. If the weight of the holocellulose is desired, the holocellulose would be filtered on a tarred fritted disc glass thimble, wash with acetone, vacuum oven dry at 105oC for 24 hours, then place in a desiccator for an hour and weigh. The holocellulose should not contain any ltelo:
ignin and the lignin content of holocellulose should be determined and subtracted from the weight of the prepared holocellulose.
3.2.3.3 LIGNIN
Procedure
Approximately 0.2 g of ground vacuum-dried sample into a 100 ml centrifuge tube. To the sample in the 100 ml centrifuge tube, 1 ml of 72% (w/w) H2S04 for each 100 mg of sample was added. Stir and disperse the mixture thoroughly with a glass twice, then incubate the tubes in a water bath at 30oC for 60 min. 56 ml of distilled water added. This results in a 4% solution for the secondary hydrolysis. 1 ml fucose internal standard was added (this procedure is required only if five sugars are to be analyzed by HPLC. as a part of the analysis). then Autoclaved at 121oC and 15 psi, for 60 min. the samples was removed from the autoclave and filter off the lignin, with glass fiber filters (filters were rinsed into crucibles, dried and tarred in crucibles using suction, keeping the solution hot. Wash the residue thoroughly with hot water and dry a 105oC overnight.
Then move to a desiccator, and let it sit an hour and Weigh Calculate Klason lignin content from weights.
3.2.4 DECRYSTALLIZATION
After the pretreatment step was applied to the three samples, for the first experiment using the 5 mm size sample, 100 g each of the samples were mixed with 125 cm of 80% concentrated sulfuric acid to effect decrystallisation, bringing the ratio of the acid to that of the sawdust to 1.25: 1 this addition of the samples to the acid was done gradually to allow excess acid after each addition and this resulted in the formation of a ugly collection of a thick dark gunk.
For the Second experiment the 0.85 mm of each sample was used and 100 g of this sample was weighed out and then mixed with 125 cm3 of the 80% concentrated sulfuric acid and ugly collection of a thick dark gel resulted.
Finally, for the third experiment, the 0.2 mm sample was used. 100 g of each sample were weighed out and then mixed with 125 cm3 of 80% concentrated sulfuric acid and this also resulted in an ugly collection of thick dark gel.
3.2.5 HYDROLYSIS
This involved two (2) steps i.e. the first and the second hydrolysis. These were done each for the three (3) experiments.
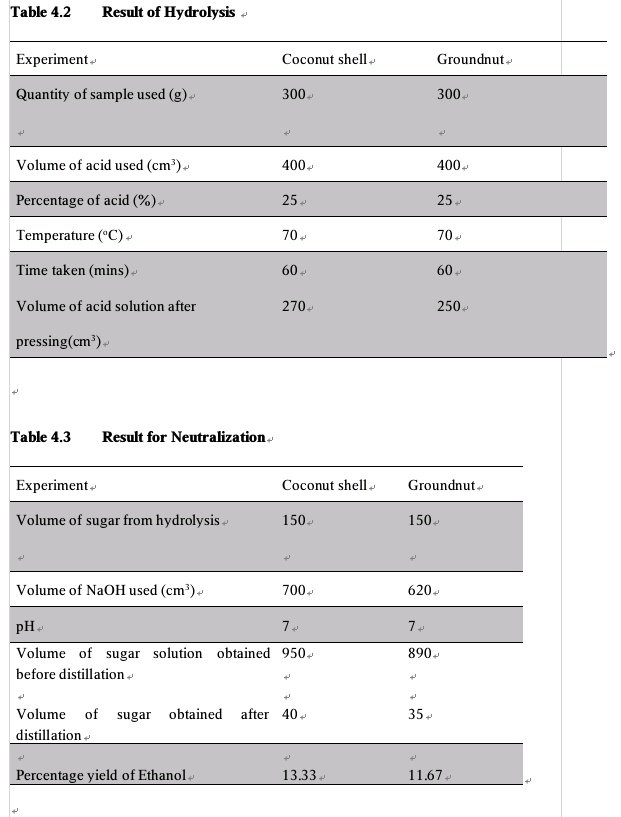
In the first hydrolysis, after decrystallization, the resulting gelatinous mass was thoroughly mixed and was diluted with 166.6 cm3 of water to reduce the acid concentration to 30% the mixture was then heated to a temperature of 90°C for 60 minutes with continuous stirring to effect hydrolysis. This was done for the three experiments. After which the acid sugar solution was then separated from the remaining gelatinous mass by pressing and of which the first experiment yielded 214.3 cm3, the second experiment yielded 268.6 cm3 and the third experiment yielded 342.2 cm3 of the acid sugar solution.
For the second hydrolysis, the residual solids from the first hydrolysis were each subjected to a second hydrolysis. So, the residual solids were each mixed with 80% sulfuric acid to effect the second hydrolysis and this resulted in the formation of another thick dark gel each and were each diluted to a concentration of 30% with water. The mixture for each of the sample were each heated to a temperature of 70°C for 50 minutes and the resulting gel were each for the three experiment were pressed out to obtain second acid- sugar solution from the first and the second hydrolysis were combined.
3.2.6 NEUTRALIZATION
After the hydrolysis, the solutions from the experiments were each neutralized with the use of calcium oxide. The calcium oxide was gradually added to ensure complete and accurate neutralization. After some addition the neutralization was confirmed by the use of red litmus paper which gave no color change at this point confirming the pH to be 7. the three thick white mixture from the three experiments were each filtered using suction filtration process and a clear yellowish liquid was obtained for the first experiment, a light green for the second and a light yellowish liquid the third experiment.
The pH was further reducetelo:
d to 4.5 by adding 0.1 molar concentrated tetraoxosulphate (vi) acid.
Equation of neutralization
H2SO4 + 2NaOH Na2SO4 +2H2O
3.2.7 FERMENTATION
In this process, the yeast was first prepared and this was done each for the three (3) experiments. The preparation was done 2 hours before the time of addition. 20 g of a baker's yeast (Saccharomyces Cerevisae) was weighed and then poured into l00 cm3 of water in a 500 cm3 measuring cylinder and this brought the yeast cream to about 200 cm3 potassium phosphate was added to facilitate the growth of microorganism. The yeast was added to the sugar solution and was left to stand for 72 hours. A test with felling solution was carried out on each of the experiment by the addition of about 2 cm3 of the sugar solution with the felling solution o which reddish- brown coloration confirmed the presence of sugar.
Equation of fermentation
nC6H12O6 yeast nC2H5OH + nCO2(g)
3.2.8 DISTILLATION
The fermented broth was put into a 250 cm3 flat bottom flask and heated with the heating mantle. The top of the flask was attached to a quick fit apparatus and a thermometer which has a constant supply of water flowing in and out. Ethanol being more volatile than water left first at the temperature of 78.5°C which was kept constant with the aid of the thermometer and with the regulator.
The ethanol obtained from the distillation of the broth was tested with some granules of sodium metal of which resulted in vigorous bubbles, confirming the presence of ethanol. Re-distillation was carried out using fractional distillation in order to obtain a higher concentration of ethanol which resulted in 34.1 cm3 for the sample size 5 mm, 55.0 cm3 for sample size 0.85 mm and 75.3 cm3 for sample size 0.20 mm.
Calculation for the percentage yield
%yield = volume of ethanol (cm3) x 100
Mass of sample (g)
3.3 TEST FOR ETHANOL
25% of iodine (blue black) solution was drop in test tube, then 10 drop of ethanol produce was added and shaked vigorously, the rest for 2 minute which is now followed by addition of 10 drops of NaOH, the yellow colouration observed shows the present of ethanol.
- RESULTS
CONCLUSION
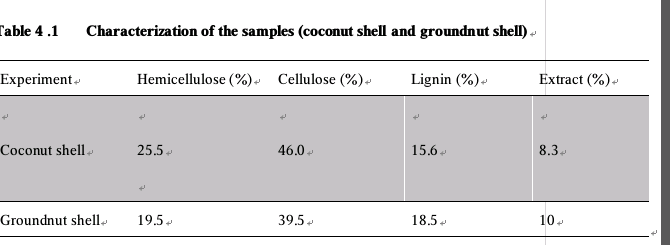
Ethanol was successfully produced from coconut and groundnut shell with the aid of saccharomyces cerevisiae as yeast for the fermentation using the mentioned approach and experimental procedure. From the result in table 4.1, It has been found that coconut shell and groundnut shell can be used to produce ethanol and Coconut shell gave a higher yield of ethanol than groundnut shell with a difference of 2%.
The utilization of abundant and renewable resources like groundnut and coconut shell to meet our future need through the production of cellulosic ethanol by concentrated acid hydrolysis will not only solve economic problems of disposal and pollution but will reduce our dependence for fuel, and above all, will save the human race from the possible risk associated with global warming. So it is therefore become unarguably necessary for government to coordinate and develop industries for the production of cellulosic ethanol from coconut shell and groundnut shell.
RECOMMENDATIONS
i The coconut shell and groundnut shell can be blended together or with other feedstock to see if a better yield could be achieved.
ii The effect of other parameters should be considered on the ethanol yield such as pH.
iii The ethanol yield should be characterized and compared with other ethanol produced from different biomass.
iv Optimization of the bio-ethanol production using various statistical analyses should also be studied.
Congratulations @telo! You received a personal award!
Click here to view your Board of Honor
Congratulations @telo! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!