Europe Biosimilar Market Report: Industry Size, Share, Growth & Analysis 2024-2032
Biosimilar Market in Europe 2024-2032
According to IMARC Group's report titled "Biosimilar Market in Europe Report by Molecule (Infliximab, Insulin Glargine, Epoetin Alfa, Etanercept, Filgrastim, Somatropin, Rituximab, Follitropin Alfa, Adalimumab), Indication (Auto-Immune Diseases, Blood Disorder, Diabetes, Oncology, Growth Deficiency, Female Infertility), Manufacturing Type (In-house Manufacturing, Contract Manufacturing), and Country 2024-2032", the report presents a thorough review featuring the market share, growth, share, trends, and research of the industry.
How Big is the Biosimilar Industry in Europe?
The biosimilar market in Europe size reached USD 11,849.5 Million in 2023. Looking forward, IMARC Group expects the market to reach USD 53,222.9 Million by 2032, exhibiting a growth rate (CAGR) of 17.6% during 2024-2032.
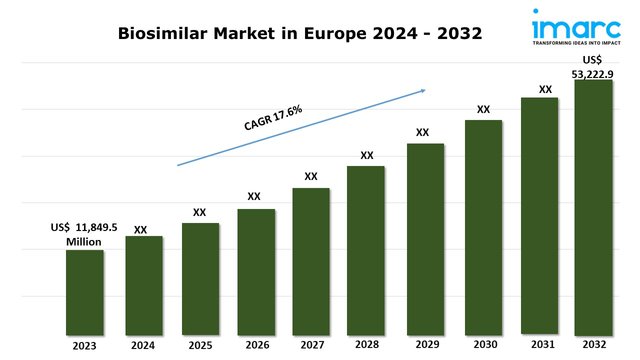
Biosimilar Market in Europe Trends:
The European market is being driven by the growing need for affordable biologic medicines. Additionally, a number of popular biologics' patents have expired, creating opportunities for biosimilar producers and encouraging competitive pricing. Additionally, the need for accessible treatment choices has increased due to the rising incidence of chronic diseases like diabetes, cancer, and autoimmune disorders, which has supported the use of biosimilars.
Furthermore, as payers and healthcare providers become more aware of the financial advantages of biosimilars, their acceptability and incorporation into treatment regimens will grow. The market is expanding as a result of patients' and healthcare professionals' increased knowledge of the efficacy and safety of biosimilars. The market is also being stimulated by government and corporate sector activities to promote biosimilars through reimbursement policies and educational programmes. High-quality biosimilars are being produced thanks to ongoing improvements in biotechnological procedures and manufacturing efficiency, which is accelerating their market penetration.
Biosimilar Market in Europe Scope and Growth Analysis:
The market's reach is expanding as a result of the growing emphasis on personalised medicine, which emphasises the demand for a variety of biosimilar medicines that address particular patient requirements. Growing healthcare infrastructure throughout Europe, especially in the region's growing economies, creates an ideal environment for the expansion of the biosimilar market. Furthermore, a strong biosimilar pipeline is being developed and commercialised thanks to pharmaceutical companies' strategic alliances and collaborations. The favourable regulatory environment, which is marked by expedited approval procedures and post-marketing monitoring, improves market credibility and expansion opportunities.
Furthermore, creativity and efficiency in product development are being boosted by the incorporation of cutting-edge technology like bioinformatics and next-generation sequencing in the creation of biosimilars. Furthermore, new biosimilar candidates are being discovered as a result of growing governmental and private sector investments in research and development. Biosimilars are expanding their market reach because of their competitive pricing as compared to their reference biologics, which makes them a desirable choice for healthcare systems with limited funds.
For an in-depth analysis, you can refer free sample copy of the report: https://www.imarcgroup.com/europe-biosimilar-market/requestsample
By the IMARC Group, the Top Competitive Landscapes Operating in the Industry:
- Novartis
- Pfizer
- Teva
- Celltrion
- Merck Sharp & Dohme
- Samsung Bioepis
- Eli Lilly
- Accord Healthcare Ltd.
- Amgen
- Boehringer Ingelheim
- Hexal Ag
- Apotex
- Stada Arzneimittel Ag
- Ratiopharm
- Mylan
Biosimilar Market in Europe Segmentation:
The market report offers a comprehensive analysis of the segments, highlighting those with the largest biosimilar market in Europe share. It includes forecasts for the period 2024-2032 and historical data from 2018-2023 for the following segments.
Breakup by Molecule:
- Infliximab
- Insulin Glargine
- Epoetin Alfa
- Etanercept
- Filgrastim
- Somatropin
- Rituximab
- Follitropin Alfa
- Adalimumab
Breakup by Indication:
- Auto-Immune Diseases
- Blood Disorder
- Diabetes
- Oncology
- Growth Deficiency
- Female Infertility
Breakup by Manufacturing Type:
- In-house Manufacturing
- Contract Manufacturing
Breakup by Country:
- Italy
- Germany
- United Kingdom
- France
- Spain
- Rest of Europe
Ask Analyst For Customization: https://www.imarcgroup.com/request?type=report&id=1023&flag=C
Key highlights of the Report:
- Market Performance (2018-2023)
- Market Outlook (2024-2032)
- COVID-19 Impact on the Market
- Porter’s Five Forces Analysis
- Strategic Recommendations
- Historical, Current and Future Market Trends
- Market Drivers and Success Factors
- SWOT Analysis
- Structure of the Market
- Value Chain Analysis
- Comprehensive Mapping of the Competitive Landscape
Note: If you need specific information that is not currently within the scope of the report, we can provide it to you as a part of the customization.
About Us:
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145