Classification of Polymer
What are Polymers?
Polymers are long chains, giant organic molecules are assembled from many smaller molecules called monomers. Polymers consist of many repeating monomer units in long chains, sometimes with branching or cross-linking between the chains.
Classification of Polymers
Polymers cannot be classified under one category because of their complex structures, different behaviors, and vast applications. We can, therefore, classify polymers based on the following considerations.
Classification of Polymers Based on the Source of Availability
There are three types of classification under this category, namely, Natural, Synthetic, and Semi-synthetic Polymers.
Natural Polymers: They occur naturally and are found in plants and animals.
Examples: starch, protein, cellulose.
Semi-synthetic Polymers: They are derived from naturally occurring polymers and undergo further chemical
modification.
Examples: silicones, and cellulose derivatives.
Synthetic Polymers: These are man-made polymers. Plastic is the most common and widely used synthetic
polymer.
Examples: polyethylene, synthetic rubbers, nylon, etc
Classification of Polymers based on the Structure of the Monomer Chain
This category has the following classifications:
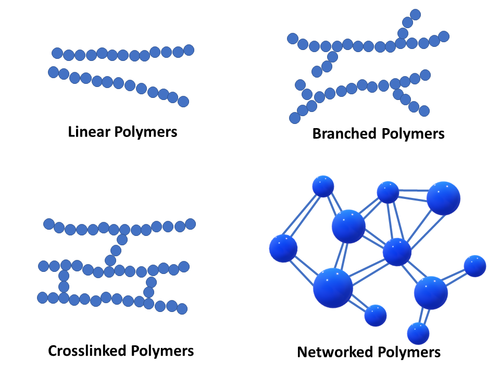
Linear Polymers: The structure of polymers containing long and straight chains falls into this category.
Example: PVC.
Branched-chain Polymers: When linear chains of a polymer form branches, then, such polymers are categorized as
branched-chain polymers.
Example: low-density polymers.
Cross-linked Polymers: They are composed of bifunctional and trifunctional monomers. They have stronger
covalent bonds in comparison to other linear polymers.
Examples: Polyester fiberglass, polyurethanes
Other Ways to Classify Polymers
Classification Based on Polymerization
Polymerization
Polymers are huge chains or sometimes even 3D structures of repeating units known as monomers. The monomer is the basic unit of a polymer. These monomers can bond to each other on each side, potentially forever.
So this reaction of combining these monomers to form long chains or three-dimensional networks is known as polymerization. Broadly polymerization can be classified into two categories,
Addition Polymerization
This is also called chain growth polymerization. As the name suggests addition polymers form when an addition reaction occurs. The repeating monomers form a linear or branch structure depending on the type of monomer. During additional polymerization, the monomers rearrange themselves to form a new structure. But there is no loss of an atom or a molecule. Again there are four types of addition polymerizations which are
Free Radical Polymerization:
Cationic polymerization:
Anionic Vinyl Polymerization
Coordination Polymerization
Condensation Polymerization
This is also called step-growth polymerization. Condensation polymers form from the step of growth polymerization. Here when molecules of monomers react to form a bond they replace certain molecules. These molecules are the by-product of the reaction. In most cases, this by-product is a water molecule.
The type of polymers that result from condensation polymerization depends on the monomers. If the monomer has only one reactive group, the polymers that form have low molecular weight. When monomers have two reactive end groups we get linear polymers. And monomers with higher than two reactive groups result in a polymer with a three-dimensional network.
Polyester and nylon are two common condensation polymers. Even Proteins and Carbohydrates are a result of condensation polymerization.
Classification Based on Monomers
Homopolymer
A homopolymer is a type of polymer that is made from identical monomers. Every basic unit of the polymer is similar to each other. A homopolymer is produced by the polymerization of the same type of monomers.
Heteropolymer or co-polymer
A heteropolymer is a type of polymer that is made of two or more different types of monomers. If two monomers are involved in the polymerization process, the end product is called a copolymer. The copolymer is a type of heteropolymer. There are several types of copolymers.
Alternating Copolymers
Random Copolymers
Block Copolymers
Graft Copolymers
Classification Based on Molecular Forces
Thermoplastics
Thermoplastics may take on amorphous or crystalline structures. In thermoplastics, the long chain molecules exist in the form of linear bonding but are also bonded to each other by secondary Van Der Waals forces (secondary bonds).
At a high enough heat, the excitation of the molecular chains is enough to overcome this binding force and they are free to move over one another thereby creating a viscous liquid. The secondary bonds can be envisaged to have melted. The glass transition (Tg) temperature can be envisaged as the temperature at which the secondary bonds melt.
When the polymer is cooled the secondary forces once again dominate and the molecular chains revert back to a restricted state. This means that thermoplastics can be melted and remelted allowing them to be easily recycled.
Thermosetting
In thermosetting plastics, the long-chain molecules exist in an amorphous network with cross-linked bonding. This means that the long molecular chains are attached to each other by covalent bonds. The formation of these cross-links is known as curing.
Cross-linking sets the molecular chains in place and therefore means that a thermosetting plastic cannot be remelted but will instead decompose upon being heated to a temperature above the Tg.
Cross-linking inhibits molecular arrangement into an ordered crystalline structure meaning that thermosetting polymers only exist in the amorphous state.
Elastomers
In elastomers, long molecular chains exist in the form of amorphous linear bonding with occasional cross-linking.
At room temperature the level of excitation of the chains has already overcome the secondary Van Der Waals bonds, however, the cross-links that exist in the structure act to revert the elastomer back to its original form following deformation.