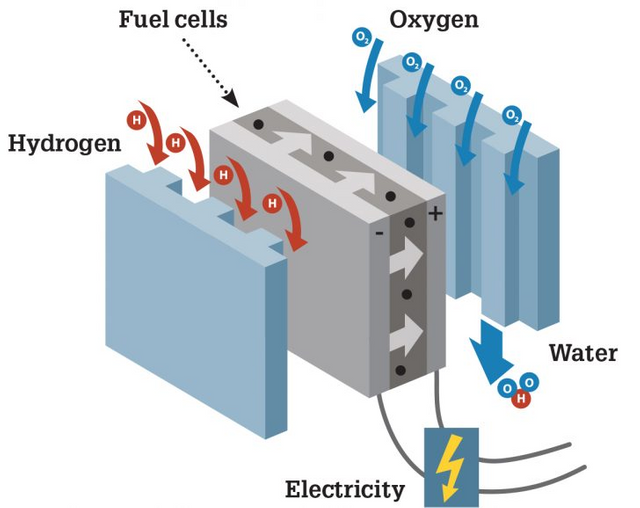
Did you know a Cell Fuel?
Did you know a Cell Fuel?

References
Spiegel, Colleen. Designing and building fuel cells. Vol. 87. New York: Mcgraw-hill, 2007.


References
Spiegel, Colleen. Designing and building fuel cells. Vol. 87. New York: Mcgraw-hill, 2007.
This post has received a 23.71 % upvote from @boomerang.