A Day In the Life of a Chemist - Making Therapeutic Drugs
What’s up steemit community, I’m going to walk you guys through a typical day for me as a scientist, particularly with synthesizing organic molecules. The project that I’m leading is aimed at making a class of chemical compounds called the Teleocidins, these compounds are found naturally growing from soil bacteria. However, it’s very complicated to extract and isolate these compounds so we decided to make them in our lab. I’ll show you guys what the structures of these compounds look like so you guys can get an idea of what I’m talking about.
First though, before I start my day, I usually drink cold brew coffee or Yerba Mate tea from the gourd, it just depends on how I’m feeling. This is a picture of some nitro coffee I bought earlier from the farmers market, first time having nitro and I must say its delicious! As you can see it pours like a nice thick porter or stout beer with a nice frothy head.
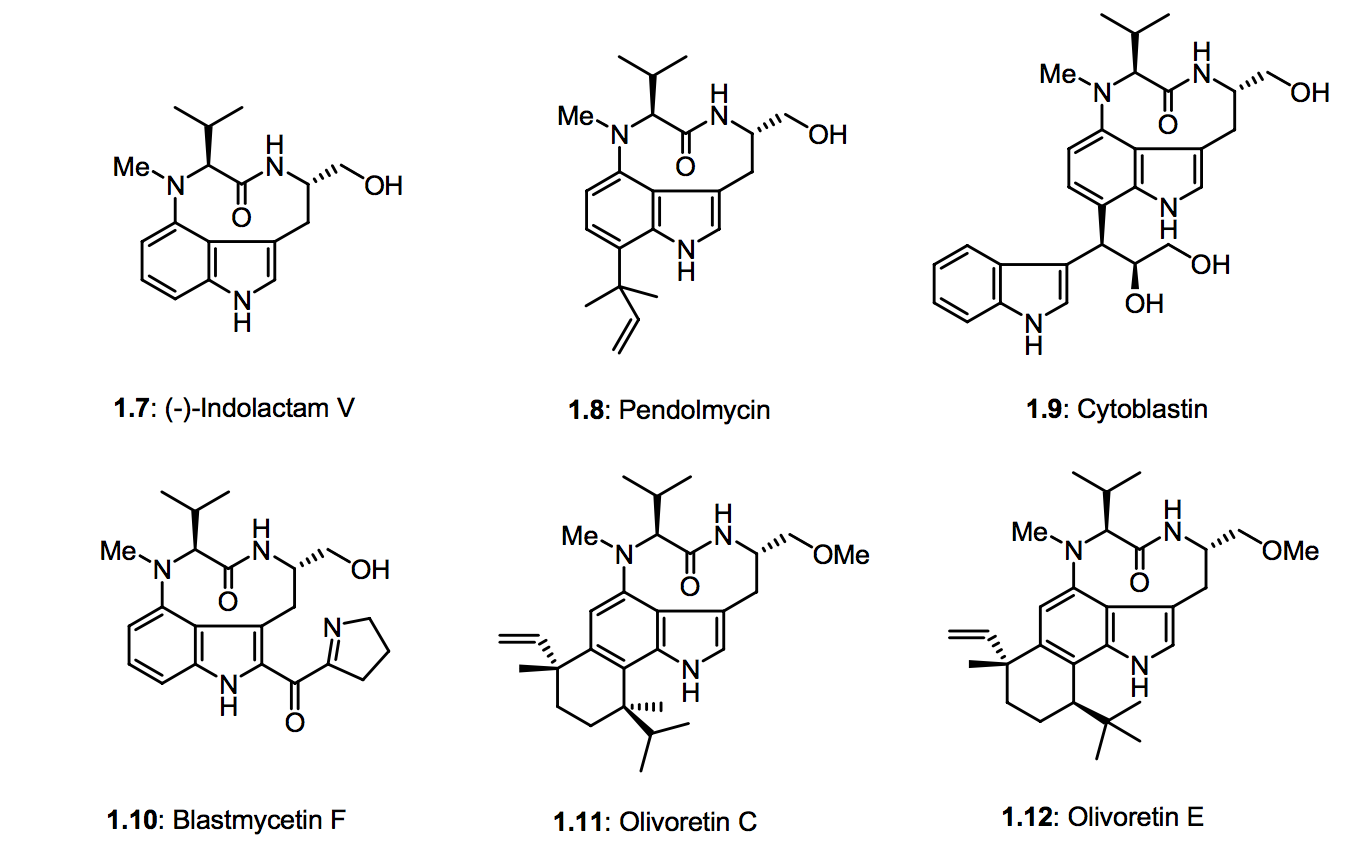
After I drink my coffee, I usually delve into my lab notebook, keeping a up-to-date notebook saves time and energy, and is the bread and butter to my success in research. I try to write everything that's meaningful down but sometimes it can get a little messy. But here's a snippet of the compounds I was referring to a.k.a the teleocidin natural products I'm making. I have successfully published and made compounds 1.7 and 1.8 respectively. I'll post a link to my publication toward the end of my article.
For the most part, throughout my day I spend it doing 'work-ups' to reaction that will potentially be tested on cancerous cells. 'Work-up' just means the process of getting rid of any impurities and side products. For example, here's a closeup of one the reactions I ran last night. The reaction is diluted with dichloromethane so its looks like a brownish oil. As you can see in the photo, I want to get rid of the excess white solids that formed during the reaction, its most likely unreacted material and impurities...basically the things I don't want.
One good method that most organic chemist use is called extraction, this takes into account the differences between organic and non organic. Sense I work with organic compounds I can easily isolate them by using a mixture of water and some sort of organic solvent system. In the picture I have posted, I'm using dichloromethane (DCM) and water. The bottom layer is DCM + product and the top layer is water + impurities + unwanted salts, the reason how I know which is which because DCM has a higher density than water so I'd expect it to float to the bottom as shown.
Once I successfully isolate the organic material from the bottom, I'll then collect in a round bottom flask where I'll attempt to evaporate off the excess organic solvent so I can get a much more concentrated solution for further isolation. Here's a picture of the equipment we use to get rid of solvents. Its called a rotovap, it uses a vacuum pump to create decrease the pressure and ultimately change the evaporation point of the solvent. Under normal atmosphere DCM evaporates very quickly so I probably don't need to use it, but it helps speed the process up by a lot.
After all the organic solvent is evaporated I run a TLC, which is just a visual technique used to see what compounds are present in the concentrated mixture. In the photo we see that I circled my product, and the spots on top are just impurities that still reside in the mixture.
To further isolate my product, I just use a liquid TLC or column, its basically the same thing as running a TLC except much bigger, check out the picture below to see what a column look like!
Once the spot is completely isolated it should be dried thoroughly using a vacuum pump under reduced pressure, this will make my product form crystals if its indeed pure. Take a look at how cool it looks when its pure!
To be extra thorough in the process I usually do one more step, which is take a nuclear magnetic resonance (NMR) test on it to see if all my peaks are present. A NMR is a huge magnet that shoots electrons at the my compound and returns information back in the form of peaks which correspond to the hydrogen count in essence. Here's what a good NMR print out should look like!
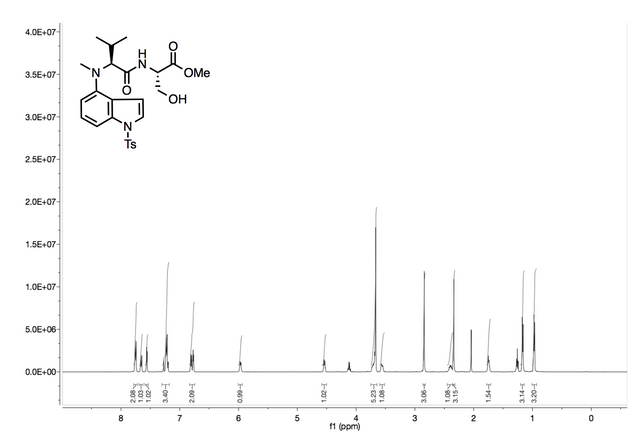
I know some of this might go over your head, but take a step back and just appreciate the process, this was a week long reaction so having patience is key when your making compounds that will be used to study cancer. If you guys like my post and like the content please upvote it and resteem with love!
Here's a link to my publication on my research!
http://pubs.acs.org/doi/abs/10.1021/acs.orglett.6b00614
Steem on friends!
We all know what you're really doing in there. I'm gonna start calling you "Walter Black".
Awesome work, thanks for sharing!
@things, read this!