A new "bed-of-nails" nano-surface selectively rips apart bacteria and leaves animal cells alone. This material could be used in medical devices and implants to prevent infections
‘Bed-of-Nails’ Surface Physically Rips Bacteria Apart
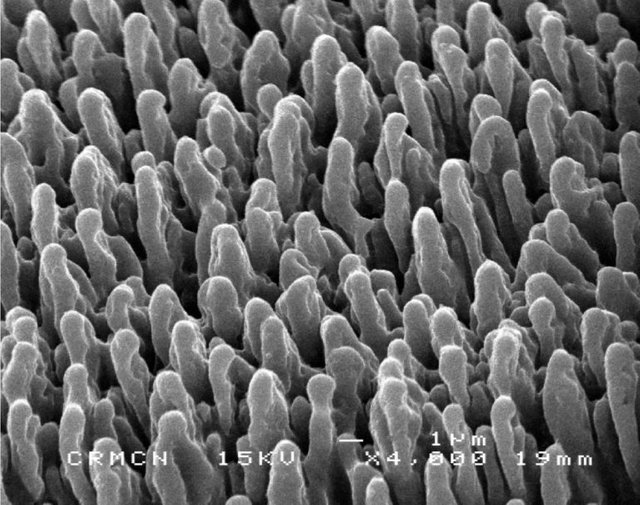
Black silicon. (Credit: Sedao (2009)/Wikimedia Commons)
Surfaces that discourage bacterial growth are in high demand, particularly as more patients require catheters and implants. Last month, we reported on a new anti-bacterial surface that incorporates molecular compounds that can block bacteria’s ability to communicate and form biofilms, which interferes with their capacity to resist antibiotics and coordinate an assault on the host.
On an implant, human cells and bacterial cells compete to colonize the surface. Obviously, any surface that can give the upper hand to human cells is preferable. So, an international team of researchers used “nanotopology” to devise a surface that physically rips apart bacteria while leaving human cells alone.
The team was aware of prior research on dragonfly wings, which are composed of bactericidal nanostructures. A synthetic material with similar bactericidal properties is called black silicon. As shown in the image above, black silicon is made up of tiny spikes, reminiscent of a bed of nails. Because bacteria are very tiny, these spikes place tremendous mechanical stress on them, and they rupture. But human (i.e., eukaryotic) cells are gigantic by comparison. Like a human laying on a bed of nails, these cells should, in theory, be just fine.
To test this, the team coated surfaces with either black silicon (bSi) or regular silicon (Si), and pre-infected them with either Pseudomonas aeruginosa or Staphylococcus aureus, both human pathogens. Then, they added cells from monkey kidneys (called COS-7 cells) to determine how they fared in the presence of pathogens with or without black silicon. (See figure below.)
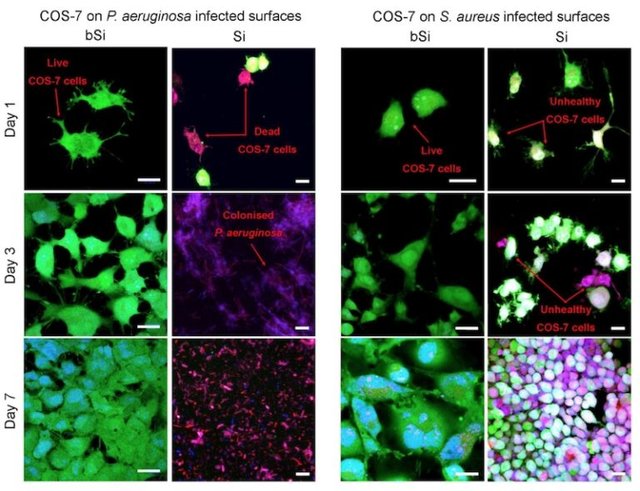
As shown above, the monkey cells (colored green) had a hard time growing in the presence of bacteria if there was no specialized black silicon surface. P. aeruginsoa completely precluded the monkey cells from growing on the regular silicon surface, while S. aureus made the monkey cells sickly. On the other hand, if grown on the black silicon surface, the bacteria were killed, and the monkey cells grew quite nicely. Furthermore, the black silicon surface is capable of killing both Gram-positive (thick cell wall, represented by S. aureus) and Gram-negative (two outer membranes, represented by P. aeruginosa) bacteria.
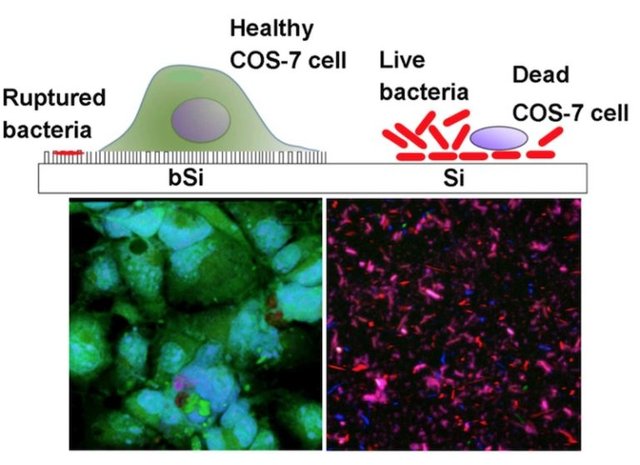
Importantly, the authors demonstrated that black silicon does not appear to cause problems for eukaryotic cells. Microscopy revealed that the monkey cells’ membranes deformed around and engulfed the tiny spikes. Additionally, black silicon was implanted into mice and did not trigger an inflammatory response.
The authors conclude that, to their knowledge, this is the first time that it has been demonstrated that eukaryotic cells can grow on a surface that had previously been contaminated with deadly bacteria. Hopefully, the authors are seeking to commercialize this technology, as it seems to be quite full of promise.
Source: Vy T. H. Pham et al. “‘Race for the surface’: eukaryotic cells can win.” ACS Appl. Mater. Interfaces. Published: 5-August-2016. DOI: 10.1021/acsami.6b06415
Full Story: https://redd.it/4wy39o
Hi! I am a content-detection robot. This post is to help manual curators; I have NOT flagged you.
Here is similar content:
http://adult-free.win/reddit/user/vilnius2013/