Both Paxlovid and Molnupiravir Failed in RECOVERY trials
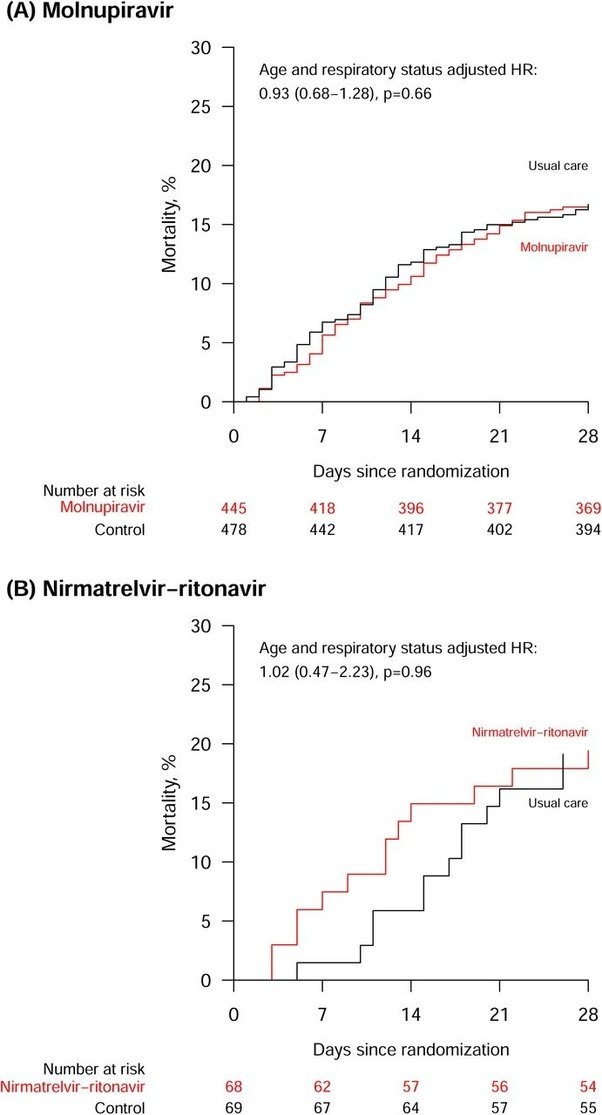
The Randomized Evaluation of COVID-19 therapy (RECOVERY) has tested antivirals for over 50,000 hospitalized COVID19 patients over the past four years at several different trial sites. In their most recent platform trials of Paxlovid (n = 137) and Molnupiravir (n = 923) administered to hospitalized patients with pneumonia syndrome mainly in the UK between January 2022 and May 2023 they found that neither antiviral reduced 28 day mortality rates compared to the standard of care. 17% of patients in the standard of care and Molnupiravir group died within 28 days of randomization even though patients assigned to Molnupiravir had lower baseline adjusted viral loads measured from nose swabs.
Similarly, 19% of patients in the standard of care and Paxlovid groups (i.e. nirmatrelvir-ritonavir) died within 28 days of randomization even though the intervention group once again had lower baseline adjusted viral load measured from nose swabs. The standard of care included administration of corticosteroids and other antivirals such as remdesivir and sotrovimab. 83% of patients in the Molnupiravir trial and 85% in the Paxlovid trial were fully vaxxed.
In these two reported evaluations from the RECOVERY trial, among patients admitted to hospital for severe COVID-19, neither molnupiravir nor nirmatrelvir-ritonavir was found to reduce mortality, duration of hospitalisation, or the risk of being ventilated or dying for those not on ventilation at baseline. However, both comparisons lacked statistical power to exclude modest differences in these outcomes.
This is not the first RCT in which Paxlovid failed to produced a statistically significant clinical benefit over the control group. Paxlovid flopped in another RCT, published in the New England Journal of Medicine, which found that it reduced the median time to sustained alleviation of all signs and symptoms by only one day less than the placebo group.