Chemistry Express: Recognizing Valence Electrons

Following the previous post about electron orbitals, today we will find out how to identify the valence electrons of elements by looking at a periodic table pattern.
Pattern for the number of valence electrons
Valence electrons are the electrons of the outermost energy shells, which participate in the formation of chemical bonds and are responsible for the chemical properties of an atom. The outermost electrons are usually also the highest energy electrons, with some exceptions we will mention below.
There is an easy trick to find out the number of each element's valence electrons just by looking at the periodic table. The vertical columns of the periodic table, called groups, each present elements with the same number of valence electrons.
For example, all the elements of the first group have only one valence electron. All the elements of the second group have two valence electrons. Skipping transition metals, lanthanides and actinides (see below for exceptions), we move to the columns for elements with three, four, five, six, and seven valence electrons.
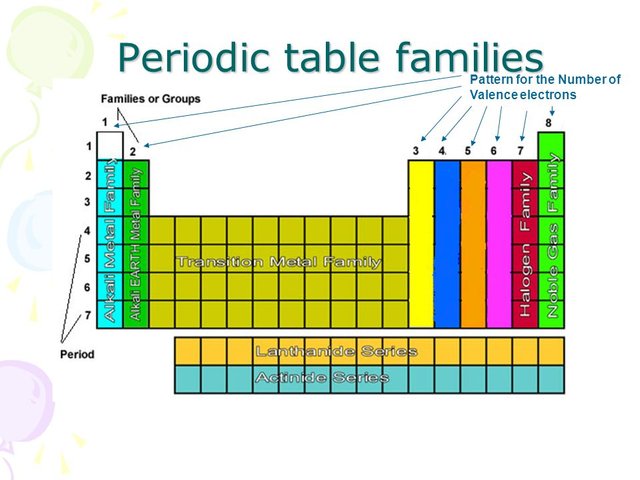
image credit
The last group is said to have eight valence electrons, but in reality, it's as if they had zero. This is because after eight electrons (or just two for the first shell) each shell is full and stable, and there are no outer electrons that can easily interact with other elements. This last group presents the noble gases, which for this reason are inert and do not take part in reactions.
And some exceptions…!
Are there any exceptions? Of course there are! These are the D-block elements - the transition metals, and the F-block elements - lanthanides and actinides. For elements of these blocks, the highest energy electrons are not in the outermost shell.
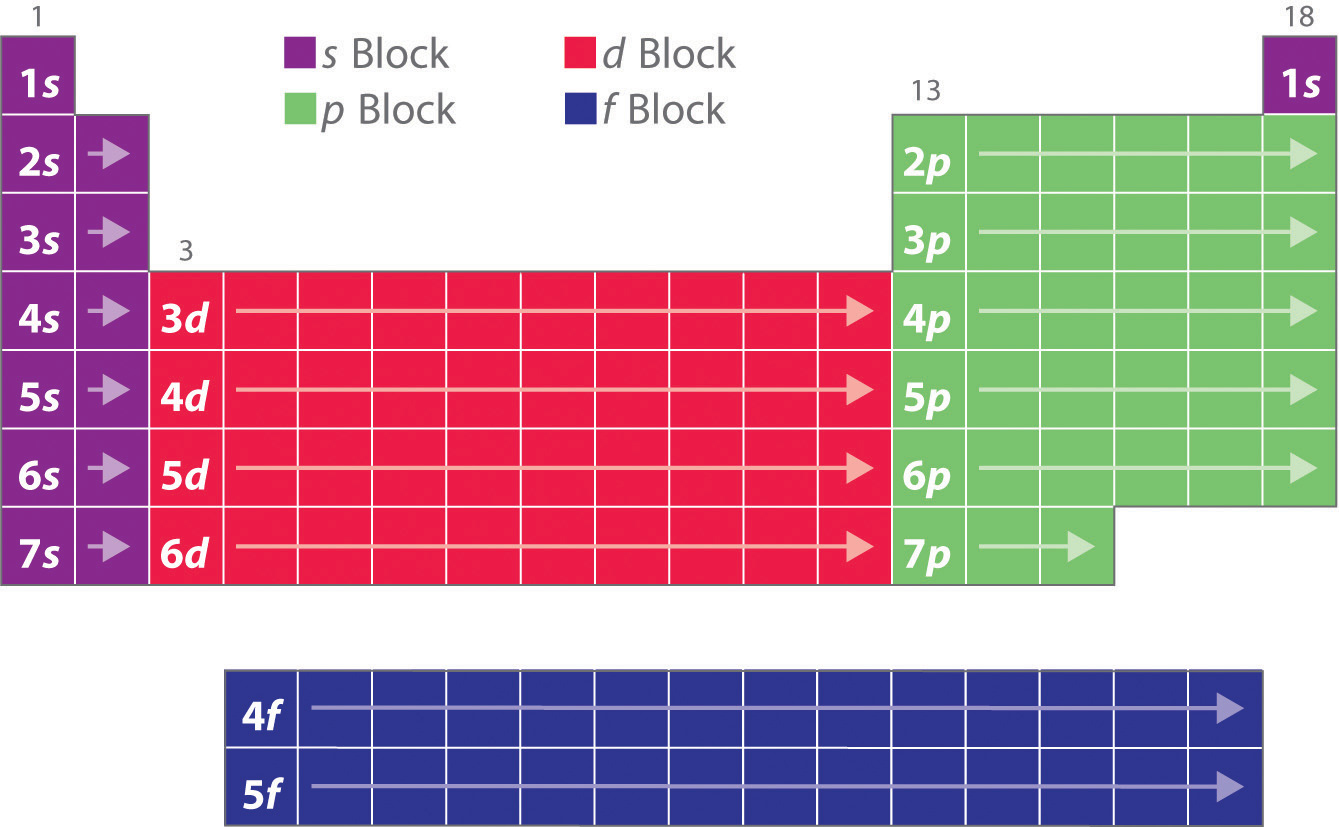
For D-block elements, the highest energy electrons are in the previous shell. So for example, iron is in the fourth period and that means that it has four electron shells. However, its highest energy electrons (the ones in d-orbitals) are actually in the third shell, not in the last one! This creates “competition” between the outermost electrons and the highest energy electrons, which renders the amount of valence electrons unstable. This in turn causes iron’s reactions to have variability and be more unpredictable!
For F-block elements, the highest energy electrons (the ones in f-orbitals) are two shells inwards, so these elements present an even more anomalous behavior and get involved in complex reactions!
In the following lessons, we'll learn how to group elements based on common characteristics and identify general trends using the periodic table.
Thank you for reading!

That is a great and clear way to promote chemistry on Steemit. We are following for some time your very nice series, and we hope you will keep on with the good work!
As a bonus, and in addition to resteeming for exposure, we are awarding you a small 10 Steem Power deposit as a thank you for creating quality STEM related postings on Steemit. We hope you will continue to educate us all!
Thank you for your help and support @steemstem! It's really motivating. I plan on continuing with the effort to make chemistry fun and approachable for everyone on steemit!
Hello my friend . Good information. Thanks for sharing Post
Please my friend I would like to get your support here your vote great honor
https://steemit.com/steemit/@mars9/steemit-the-idea-in-a-white-paper-was-published-in-march-2016-now-the-dream-is-coming-true
Thank you. You have some interesting posts in your blog. I'm following :)
This is really helpful, thank you! :D
I'm occasionally interested in chemistry myself - for no reason at all, and I have to say that it's really cool that you are sharing all these with us noobs!
Thank you! Chemistry is inherently interesting, so I totally get your point ;)
Great post! I think chemistry is really interesting, always got bad grades in high school though :P
Thanks! Many people get bad grades because they have missed out on some basics and since everything builds on these basics from there on, they don't understand. I used to have average grades in math. When I started learning it again on my own, it was a whole different story.
Yeah and I guess it's important to have a good teacher, I didn't like mine much ;)
You started a good series! I hope you will continue to summarize these aspects for everyone.
For my part, I had a similar idea. Though, at this point, I didn't want to do this as systematic as you but to point out single topics in an (hopefully) easy understandable way.
Thank you. I checked your blog out and I liked your post about the colored flames. I will be following you!
Good work friend
thank you :)
welcome