Oxygen Demand : Biochemical and Chemical

DEFINITION OF TERMS:
(All terms have units of mg O2/L)
BOD (Biochemical Oxygen Demand) – the amount of oxygen utilized by microorganisms in oxidizing (consuming) carbonaceous and nitrogenous organic matter.
CBOD(Carbonaceous Biochemical Oxygen Demand) – BOD where the electron donor is carbonaceous organic matter.
NBOD (Nitrogenous Biochemical Oxygen Demand) – BOD when the electron donor is nitrogenous organic matter.
ThOD (Theoretical Oxygen Demand) – the amount of oxygen utilized by microorganisms in oxidizing carbonaceous and/or nitrogenous organic matter, assuming that all of the organic matter is subject to microbial breakdown, i.e., it is biodegradable.
BOD5 (5-day Biochemical Oxygen Demand) – the amount of oxygen consumed (BOD exerted) over an incubation period of 5 days; the standard laboratory estimate of BOD. The 5-day BOD utilizes the notation y5, referring to the BOD exerted (y) over 5 days of incubation.
BODU(Ultimate Biochemical Oxygen Demand) – the amount of oxygen consumed (BOD exerted) when all of the biodegradable organic matter has been oxidized. The ultimate BOD utilizes the notation Lo , referring to its potential for oxygen consumption when proceeding to complete oxidation.

COD (Chemical Oxygen Demand) – the amount of chemical oxidant, expressed in oxygen equivalents, required to completely oxidized a source of organic matter.
Organisms derive the energy required for maintenance of metabolic function, growth, and reproduction through the process of fermentation and respiration. Both organic and inorganic matter may serve as their source of energy.
Chemoheterotrophs – are organisms that utilized organic matter – C(H2O)- as a carbon and energy source and, under aerobic conditions, consume oxygen in obtaining that energy:
C(H2O) + O2 → CO2 + H2O + Δ
Chemoautotrophs – are organisms that utilize CO2 as a carbon source and inorganic matter as an energy source, and usually consume oxygen in obtaining that energy.
Example of chemoautotrophy:
Nitrification - the microbial conversion of ammonia to nitrate (with bicarbonate ion contributing CO2):NH4+ + 2HCO3- + 2O2 → NO3- + 2CO2 + 3H2O + Δ
In all these redox reactions, oxygen is consumed. Thus, BOD is a measure of strength of a water or wastewater: the greater the concentration of ammonia-nitrogen or degradable organic carbon, the higher the BOD.
SOURCES OF BOD:
Domestic wastewater and many industrial wastes are highly enriched in organic matter compared with natural waters. Proteins and carbohydrates constitute 90% of the organic matter in sewage. Sources include: feces and urine of humans, food waste from sinks, soil and dirt from bathing, washing and laundering; plus various soaps, detergents, and other cleaning products
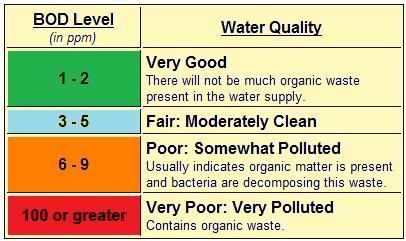
BOD MEASUREMENT/TEST
Without Seeding Method - is used if microbial populations are abundant in wastewater
Method:
After measuring the initial DO, samples are incubated at 20oC to encourage microbial activity (respiration is temperature dependent).
To ensure that oxygen is not completely depleted before the end of incubation period, aerated dilution water is added. The dilution water may also contain inorganic nutrients (e.g., Fe, N, and P) required by the microbes.
Sample pH can be adjusted and dilution water maybe added to reduce or eliminate toxicity in case of extreme pH and presence of chemicals such as heavy metals.
The BOD bioassay is conducted in the dark to inhibit oxygen production through photosynthesis should algae be present, as in the case of many samples from natural waters. After the incubation period, final DO is measured.
With seeding – is used if microbial populations are absent or present in low numbers. This case is typical in many industrial or disinfected wastes and in some natural waters. In this case, microbes maybe purchased or obtained from biological treatment plants and added to the sample as “seed”.
Method:
The BOD test is carried out by diluting the sample with oxygen saturated dilution water, inoculating it with a fixed aliquot of seed, measuring the dissolved oxygen (DO) and then sealing the sample to prevent further oxygen dissolving in.The sample is kept at 20 °C in the dark to prevent photosynthesis (and thereby the addition of oxygen) for five days, and the dissolved oxygen is measured again. The difference between the final DO and initial DO is the BOD. The loss of dissolved oxygen in the sample, once corrections have been made for the degree of dilution, is called the BOD5.
Separating CBOD and NBOD:
The standard BOD test measures both CBOD and NBOD. Occasionally, it is necessary to separate the two processes to support plant design or operation.
Method:
A chemical maybe added to the water sample to inhibit nitrification, yielding CBOD as the sole result of the assay.
- NBOD may then be determined by difference from an analysis in which no inhibitor was added. In an inhibited assay, results are reported as CBOD; without inhibitor addition, results are reported as BOD.
Source: Lecture from
Introduction to Wastewater Treatment
Lesson 4