Really, The Glass is A Moving Fluid ?? [Chemistry]
Hello dear steemian, hopefully everything still healthy for reading steemit post, tonight i will share a post base on Silica.
Glass (SiO2)
 Source :pixabay
Source :pixabay
Glass is a complementary household processed in chemical industry, many of household appliances made from the glass such as, cup, plate, glass for cabinets, for windows etc. From physical point of view the glass is an easy break material when i hit with a hard object. but if assessed of chemistry terms of the glass is a moving fluid. Or we can imagine the glass like an ice, ice is frozen water has hard physical and can be broke by bumping it into hard object. Basically the ice is water ie liquid substance. To make reader to easier understood i attached a video of glass processing.
Example a glass in our kitchen was hard material, if it fell to the floor or pressed then it will be destroyed, because it was frozen. but in making process it was elastic properties, can be inflatable and shaped as desired. Why ? Because characteristic of Silica (SiO2). Therefore, lets to know more about silica.
Glass is transparent material composed of inorganic oxide compounds of alkali or sand as result of decomposition and it is non-volatile compounds. It has special properties due to the silica influence (SiO2). Silica is a natural chemical contained in nature with chemical formula SiO2, it can be found in quartz sand and it has melting point of 1,713oC. Chemically, the glass is a quartz because it characteristic was transparent material, but physically it is a "cold liquid" because its constituent structure is far from each other like liquid but it is solid.
Glass Forming
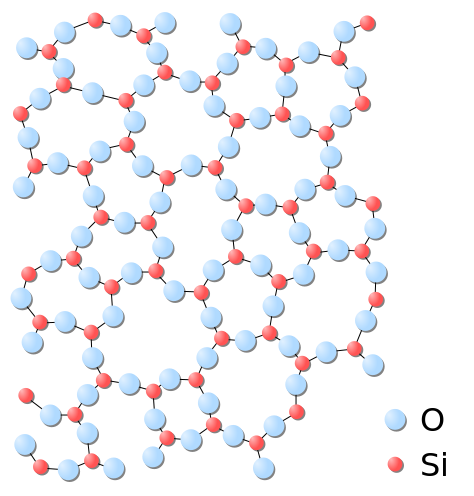 Source :wikipedia
Source :wikipedia
The silica (SiO2) has melting point of 1,713oC, when heating time the silica will fuse so it can be formed easily, when it has cooled it be hardened or frozen immediately, the glass can be formed in a hot state, due to the influence of silica melting point makes silicone elastic and easily shaped as desired, so that when temperature drops the glass will instantly become harden. There are some chemicals that can to manufacture glass such as Silica (SiO2), Sodium Oxide (Na2O), Magnesium Oxide (MgO), Sodium Tetraborate (Na2B4O7), Aluminium Oxide (Al2O3) and Lead Oxide (PbO) Each chemical raw material has different advantages such as using of sodium oxide can increase the expansion when manufacturing process and using of lead oxide can make glass in high quality or called as "flint". The process of glass making includes several stages they are, heating, forming (shaping) and blowing. The homogeneous feedstock inserted to the furnace and undergoes reaction, the chemical reaction of glass forming can be written with equation:
Decomposition Reaction
Na2CO3 → Na2O + CO2
CaCO3 → CaO + CO2
Forming Reaction
Na2CO2 + aSiO2 → Na2O.aSiO2 + CO2
CaCO3 + bSiO2 → CaO.bSiO2 + CO2
The reaction process of glass formation occurs at high temperatures, when the silica was melting the glass can be formed. The sodium carbonate or calcium carbonate compounds decompose into sodium/calcium and carbon dioxide (CO2), so the silica (SiO2) compounds bind to Sodium/calcium to form sodium silicate by chemical formula Na2O.aSiO2.
Conclusion
Conclusion
 Source :pixabay
Source :pixabay
Glass is a clear/transparent material used in household, the glass made with silica (SiO2) element which reacted at high temperature, when the silica reaches melting point, it becomes elastic and easy to be formed(shaping), or by reaction above the glass can be formulated as Sodium/Calcium Silica or chemical formula Na2O.aSiO2.
Source
Support Scientist By Use #science tag or join @steemSTEM
Follow Me @jamhuery

thanks, good information for me