Where Does The Diamond Mined ?
Hello dear steemian, today i wrote a post about an element that has a different value, price but composed of some element,lets to the point.
 Source :kompasiana
Source :kompasiana
The diamonds and coal are composed of same elements, so some people assume both diamond and coal were present in the same mine. Therefore I will explain every process of forming both in terms of area, pressure and temperature. Chemically, these two materials are composed of carbon elements but has different structures. After readers understand the process of coal and diamond forming, the reader will understand where diamonds can be mined.
Where Coal Was Mined ?
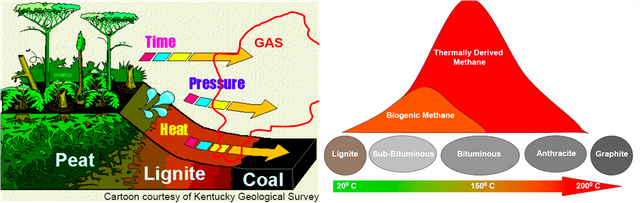
Graphite is an carbon allotrope, it comes from weathering rest of metamorphogenic process, the process of its forming begins with plant death then be a precipitate (peat), because the influence of temperature, pressure and time, the peat entry into earth layer then form to ignite rocks, over time the ignite change to be coal, its layers composed of bituminous, sub-bituminous and anthracite. The process of changing from lignite to the coal, it requires temperatures above 200°C and pressure less than 0.5 GPA, at a depth 7 to 8 KM of earth's surface. The mining of graphite can be done with open pit mining method, if the graphite deposit near of earth surface, may be by drilling method or blasting the ground with dynamite. And Underground mining method can be done by soil drilling either vertical or horizontal, the soil made as underground tunnel.
Graphite can be synthesized in laboratory scale by using alumino silicate (clay) and Carbon, its synthesis requires temperature around 900°C and pressure 1 GPA, by chemical formula graphite synthesis can be formulated.
Al2SiO3 + C => SiC + Al2O3
SiC + Heat => C (allotrope)
The reaction of aluminium silicate and carbon produces a blue crystal of silicon carbide (carborundum), the heating of carborundum and leave carbon rest (allotrope), The carbon (allotrope) evaporates at temperature 4150°C and produce the graphite. The coal forming can occur with biochemical (peat) and geochemical processes, the geochemical processes include changes of lignite to bituminous and anthracite. The layer, coal structure and magma intrusion influenced by geotectonic (pressure).
Where Does Diamond Formed ?
 Source :jairclopes
Source :jairclopes
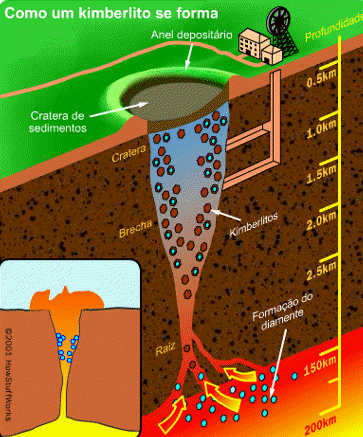
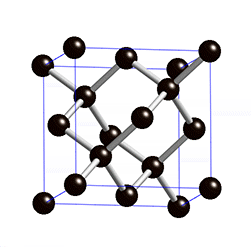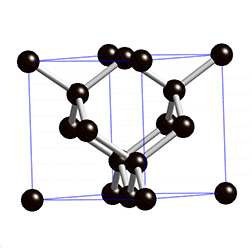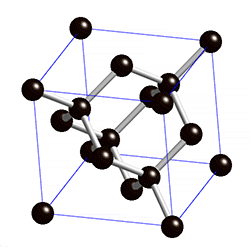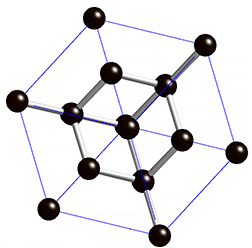
Diamond is an allotrope carbon with tetrahedral carbon structure, the diamonds formed beneath of earth surface of in liquid magma at a depth about 100-200 Km and temperature of 900-1300°C with a pressure about 4.5-6.0 GPA, The powerful magma eruption carrying kimberlite (diamond-encrusted rock) to the earth surface, the magma eruption road called as volcanic pipe, the diamond-containing volcanic pipe called kimberley (kimberlite rock), due to the continued of geological process, the diamonds on the sedimentary of volcanic pipe can transmit diamond to the alluvial deposit, this transportation called glacier or the process of water transport, So the diamonds can be mined in alluvial and kimberlite deposits. Basically, the diamonds formed deep inside of earth mantle, because eruption process of magmatic can lead diamonds to the earth surface, if we see from the process of its formation, diamonds can be mined in area where mountain eruptions have occurred as kimberlite deposits, and surrounding river areas as alluvial deposit.
Diamond is a transparent white mineral, but there are also diamonds in blue, yellow,color. The colors in diamond caused by impurities element. Chemically, the reaction of impurity element we can symbolize with alpha, The white color is the Clear Diamond or without impurities element.
Br + C => BrαC Blue N + C => NαC Yellow
Where α is Boron (Br) and Nitrogen (N), These impurities can change the diamond color to the blue and yellow, while red or pink caused by tectonic proses, and green color caused by natural radiation.

Conclusion
Conclusion
 Source :kompasiana
Source :kompasiana
The diamond and coal are made up of same element, but the formation of diamonds requires higher temperatures and pressures and deep depths, impossible the diamond formed in coal deposits, because the diamond formation requires 4.5 to 6.0 Gpa pressure, because of high pressure the structure bonds of diamond become covalent and tetrahedral, whereas coal formation requires only 0.5 Gpa pressure at a depth of 7-8 Km. The diamonds found at river base is the result of the glacier process.
Source :
Support Scientist By Using #science tag or Join @steemSTEM
Follow Me @jamhuery

I was curious about that diamand. Thanks for this informative post.
I am not so very sure about how correct this is.
But i remember i read something about diamonds found that they said was made in space without pressure.
If i dont remember wrong, one meteorite with those diamonds landed in Sibir.
But as i say, i am not 100% sure about this.
And when i did a short google search now, i did not see it (i did not look for long)
Thanks for reading, i also ever read about deposit diamond from asteroid but some research said this matter still can not be proven or weak references, so diamond deposit from the asteroid I don't input here.