Overview of The Petroleum Industry
The petroleum industry, the first commercial well drilling well opened in 1859 and two years later it starts with obtaining kerosene from petroleum. When petroleum refining is evaluated from the first application, simple distillation, to the complex processes of today, it is seen that the greatest effort is spent to meet the health and safety requirements and to provide a safe working environment. Petroleum refineries are a complex of various units. Refining is a complex mixture of hydrocarbons from some other complex hydrocarbons. High temperatures and high pressures are applied when flammable gasses and liquid products are obtained in the process. All necessary equipment temperature, pressure, corrosion, tensile strength under the supervision of experts.

Electronic technology allows operators to continuously monitor process units day and night. Computerized process control systems are available in the control rooms in each operating area. The data, graphs and interactive graphics related to the operations of the plants are displayed on the screens. The process control system allows operators to fine-tune the process, if necessary, by intervention and immediately take the result of the process change.
History

Petroleum refining has been shaped and developed according to the demands of the consumer with higher quality and more products. The first refinement was aimed at obtaining kerosene that is lighter and cheaper than whale oil. With the discovery of internal combustion engines, gasoline and diesel fuel production has begun. With aircraft fuel demand has led to the start of high-octane gasoline and jet fuel production.
| Year | Process Name | Purpose of Process | By-product, etc. |
|---|---|---|---|
| 1862 | Atmospheric distillation | Kerosene production | Naphta, tar, etc. |
| 1870 | Vacuum distillation | Crude oil fractionation | Asphalt, residue |
| 1913 | Thermal cracking | Increasing gasoline efficiency | Residues, bunker fuel |
| 1916 | Sweetening | Sulfur and smell reduction | Sulfur |
| 1930 | Thermal reforming | Raising octane number | Residue |
| 1932 | Hydrogenation | Sulfur removal | Sulfur |
| 1932 | Coking | Increasing gasoline base stocks | Coke |
| 1933 | Solvent extraction | Increasing VI oils | Aromatics |
| 1935 | Solvent devoxing | Editing the pour point | Waxes |
| 1935 | Layer polymerisation | Increasing gasoline yield and octane | Petrochemical raw materials |
| 1937 | Catalytic cracking | Higher octane gasoline | Petrochemical raw materials |
| 1939 | Visbreaking | Decreasing viscosity | Distillates and tar |
| 1940 | Alkylation | Increasing gasoline yield and octane | High octane aircraft petrol |
| 1940 | Isomerization | Alkylation raw materials | Naptha |
| 1942 | Fluid catalytic cracking | Increasing gasoline yield and octane | Petrochemical raw materials |
| 1950 | Deasphalting | Increasing cracking raw material | Asphalt |
| 1952 | Catalytic reforming | Naphta conversion | Aromatics |
| 1954 | Hydrodesulfurization | Sulfur removal | Sulfur |
| 1956 | Inhibitor sweetening | Mercaptans removal | Disulfurs |
| 1957 | Isomerization | High octane molecules | Alkylation raw materials |
| 1960 | Hydrocracking | Improving quality | Alkylation raw materials |
| 1974 | Catalytic degaxing | Editing the pour point | Waxes |
| 1975 | Residual hydrocracking | Increasing gasoline efficiency | Heavy residues |
Distillation Processes

The first refinery was opened in 1862 and simple atmospheric distillation produced kerosene, which contains tar and naphtha among its by-products.
After a short time, it was discovered that by distilling the oil under vacuum, high quality lubricating oils could be obtained. For the next 30 years the basic demand of the consumer was again gas oil. This situation was changed two events. Reduction of gas oil demand for lighting purposes by discovery of the electric and increase in diesel fuel and petrol demand due to the use of internal combustion engines.
Thermal Cracking Processes

The development of the automotive industry and the First World War led to the proliferation of gasoline-powered vehicles and, in parallel, the rapid increase in petrol demand. However, the amount of gas obtained by distillation processes is small and limited , the thermal cracking process was developed in 1913. Heavy fuels that contain large-molecule hydrocarbons are decomposed under pressure and heat and converted into small-molecule hydrocarbons to increase gasoline production and distillate fuels.
Another thermal cracking technology is coking (1933). It is a severe thermal cracking process applied to convert heavy residues into lighter products and distillates. Visbreaking (1939) is a soft form of thermal cracking process. Atmospheric or vacuum distillation residues are converted into gas, naphtha, distillates and low viscous fuel oil by thermally cracking in the absence of catalyst.
Catalytic Processes
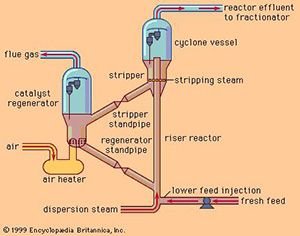
In high-compression gasoline engines, there is a need for a knock-resistant high-octane petrol engine. The quality of gasoline was improved by catalytic cracking, alkylation (1940) and polymerization processes, which were developed from the middle of the 1930s to the end and the high-octane gasoline demand was met. After, a catalytic isomerization process was developed to convert the hydrocarbons into alkylation raw materials. Process methods such as hydrocracking, steam reforming, vapor cracking and devxing were developed with catalysts improved in 1960. These catalytic processes are also the basis of the modern petrochemical industry, since double bond molecules (alkenes) can also be obtained.
Treatment Processes

Various treatment methods are used to remove non-hydrocarbon materials, impurities and other substances that affect the properties of final products or reduce the efficiency of conversion processes. Treatment is chemical reaction or physical separation. Typical examples are chemical sweetening, acid treating, klay contact, caustic scrubbing, hydrotreating, drying, solvent extraction and solvent degassing (wax removal). Sweetening is applied to crude oil before prosesting to purify from sulfur.
REFINERY PROCESSES
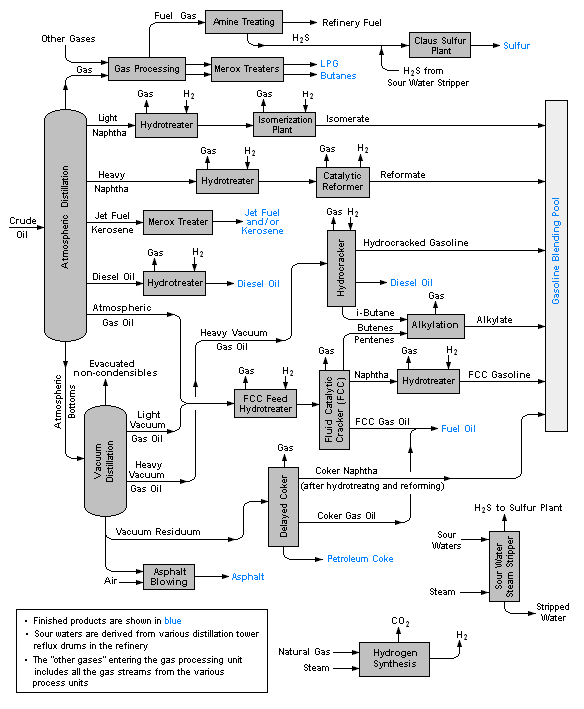
Oil refining starts with distillation or fractionation processes to separate the crude oil into hydrocarbon groups. Most of the products are then subjected to processes such as cracking, reforming, and other conversion processes to change the size and structure of the hydrocarbon molecules they contain and transformed into highly used products. The next stages are various processing and separation processes such as extraction, hydrotreating and sweetening. Whereby the undesired compounds are removed and the quality of the products is increased. In integrated raffiners, fractionation, conversion, treatment and blending operations are combined, even petrochemical processes may be included in them.
Refinery processes and operations can be grouped into five general groups.
Fractionation (Distillation)
This process is a process in which crude oil is distilled in distillation columns under atmospheric and vacuum conditions and separated into hydrocarbon groups at different boiling intervals, these are called fractions or cuts. The basic fractionation processes are atmospheric and vacuum distillation processes.
Conversion
The size or structure of hydrocarbon molecules is changed by conversion processes. These are
- Decomposition, thermal and catalytic cracking
- Unification, alkylation, polymerization and compaction
- Alteration and rearrangement, isomerization and catalytic reforming.
Treatment
The purpose of this process to prepare hydrocarbon flows in the subsequent pressing and shaping to final products. Separation or removal of aromatics and naphthenes as well as removal of impurities and impurities is also included in the treatment procedures. Treatment may be in the form of chemical or physical separation as dissolution, absorption, precipitation, drying, hydrodesulfurization, solvent deasphalting, sweetening, solvent extraction and solvent devoxing etc.
Blending and Other Processes
Blending is the process of combining and mixing hydrocarbon fractions, additives and other necessary compounds to obtain products with specific performance criteria. Blending is the latest and critical stage of refinery operations. For example, a gasoline product, is obtained by blending the components from various process units. The octane level of the mixture, the vapor pressure value, and other properties must match those specified for the intended use. In addition, minerals, waxes, greases, unsaturated and saturated gas plants, hydrogen production and MTBE factories were also grouped under this group.
Aaaah yeah @kedi
You earned yourself a 0.29$ upvote!
PS: You can earn 30% Vote-Cashback by upvoting this comment and all other post/comments by @therealwolf
Checkout: http://steem.link/introduction-wolfs-voting-bot
Nice article, great post! Oil is always necessary.
Look at my posts too @thunderland maybe follow me, if you like them.
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Yaser from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
The oil industry has come a long way since 1892. Thanks for the great pictures and explaining the process. I grew up near a refinery but never understood how involved it actually is!
You are welcome
Perfect post nice to meet you @kedi, and i wanna say i like your post ;)
Thank you @jamhuery, nice to meet you too :)
welcome @kedi, help to share chemistry science for each other ;)
I like this article, it's very interesting and complete, I'm an Oil Engineer. Congratulations for this!!!