Why You'll Die From Dehydration Drinking Seawater (and How Not to Die Quite So Fast)
There are a lot of ways to die, but getting lost at sea and dying of thirst is probably not something anyone would relish. What a crazy thing to think that we would die of thirst surrounded by water, but it's true, we can't drink (only) seawater and survive. Why we can't drink seawater and survive, though, is quite interesting.

Image Credit: Wikimedia Commons
It's All About Mineral Concentrations
Mineral concentrations in water are the key to why we can't survive on seawater. Mineral concentrations in liquids are measured several ways, but we'll use a measurement called osmolality. Osmolality measures the concentration of dissolved particles in the fluid.
Think about osmolality like this. If you put 1 teaspoon of sugar into a cup of coffee, you'll have a certain amount of dissolved sugar in the coffee. If you added two teaspoons you'll have twice as much dissolved sugar in your coffee. If you accurately measured how much sugar you used and the volume of your coffee, you'd be able to measure the osmolality of your coffee. We can do the same with any solids that dissolve in liquid, and can compute this concentration in a measurement called milliosmoles per liter, which is abbreviated mOsm/L.

Image Credit: Giphy
Humans are made up of about 61% water (1), and all of the cells and compartments contain fluids that each have varying degrees of dissolved solids in them from the minerals we ingest, such as sodium, magnesium, and potassium, just to name a few. Our body fluids, namely plasma, the watery portion of our blood, averages about 300 mOsm/L (2), or about 0.9% of dissolved solids. Seawater, on the other hand, is about 3 times more concentrated, at about 1000 mOsm/L, or about 3% of dissolved solids (3).
Don't Drink the (Sea)Water! At Least Not Too Much.
This large difference in concentrations of dissolved solids between body fluids and seawater creates the problem of why humans will die of dehydration from drinking seawater.
You see, when you have a difference of concentration of fluids separated by a semipermeable membrane (like how the blood is separated from the digestive tract), then the compartment with the lower concentration will flow into the compartment with the higher concentration in an attempt to make the concentrations equal.
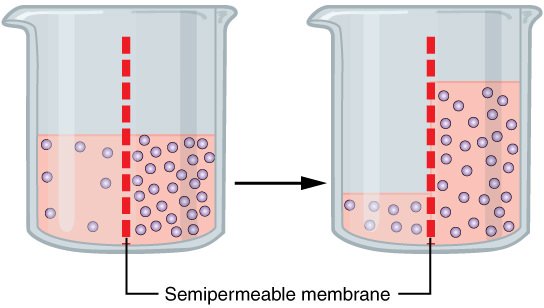
Wikimedia Commons
In the case of drinking seawater, the lower osmolality in the blood will cause fluids to flow from the bloodstream and into the digestive tract. In the image above, imagine the left side of the beaker being the blood, and the right side of the beaker is the digestive tract when you drink seawater.
This is not a problem if you drink certain amounts of seawater, and are able to counteract it with fresh water. In fact, in the accounts of the Kon Tiki, a raft that sailed across the Pacific from Chile the Polynesia, the crew experimented with drinking different percentages of seawater and found they could mix up to of 40% seawater in fresh water without any ill effects. In fact, adding seawater in percentages around 30% made them feel less thirsty than with the same amount of just fresh water, which could be an effective survival strategy for making one's water last longer if lost at sea.
But if you are capsized on the ocean with only seawater, you're better off drinking nothing than drinking seawater since, as you can see, you will become more dehydrated even more quickly and hasten your death by dehydrating your cells sooner. The moral of the story is: Don't get lost on the ocean, and if you do, take lots of fresh water, mix it with seawater to make it last longer, but never drink pure seawater.

Kon Tiki was such a great book!
That would have been such an amazing adventure. Maybe less so their second trip when there was less sea life and more oil slicks and garbage (and that was how many decades ago....don't know that I'd want to drink the 40% sea water in my water now....though I'm sure you'd do what you gotta do stuck out in the vastness of the ocean.)
Not going to lie, I prefer my ocean travels now to be in a hot tub on a cruise ship. That's the way to do it! All you can drink not-seawater! (Sure there's a little norovirus to contend with, but hey, cruise ships carry tons of bleach [literally]).
Very cool that 30% seawater seems to make you feel less thirsty. Especially since that mixture would be isotonic to your body.
I think difference in the osmotic pressure leads to dehydration which then leads to death.Thanks for this enlightment @kerriknox
increased pressure gradient created by the high minerol concentration in sea water leads to dehydration.
What if we boil the sea water and collect the water vapor to turn it into fresh water, would that help?
Another way can be through reverse osmosis if we somehow got an artificial semi-permeable membrane (possibility tends to 0). :p
However, thanks for the really amazing post @kerriknox. Very useful survival strategy in sea. And the way you explained all the this is commendable. I learnt a lot. :)
We are suffering a severe-drought here in Cape Town in South Africa. The Government has set aside funds for a project to make salt-water drinkable. Whether it will come to fruition, I don't know. Regardless, I always thought about this when I watched the movie "Water world".
Thanks for the educational post.
@rionpistorius
I only drink Brawndo, the thirst mutilator. It has electrolytes.
Wow, amazing scientific post about sea water.....in many places, fresh drinking water is a scarce commodity. Really fascinating to learn why its not possible to drink salt water.... Great post!

Good educational information should be shared so your post has been Upvoted and Resteemed. Hope more people get to read your blog
There is a saying in my local language about eating more salt or drinking salt water. If a person eats a food that has more salt content, he will end up suffering from high thirst. It can be compensated only by drinking large quantities of proper water.