Meet your microbes: Streptococcus salivarius
Have you ever wondered what bacteria live, breed, and shit in your mouth? Our oral microbiome is teeming with hundreds of different species of bacteria, but today I would like to introduce you to one of them in particular, along with our designs to engineer it into a cavity fighter: Streptococcus salivarius.
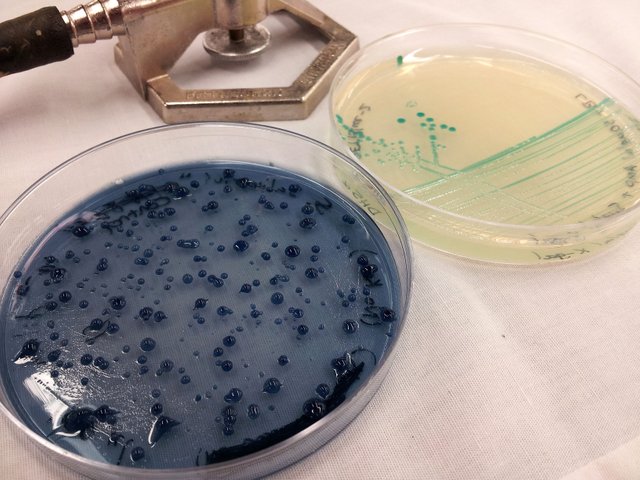
Belonging to the streptococcus family these bacteria form linear chains through division along a cellular axis; think about a microbial conga line. While these critters divide to form chains, they can also generate biofilms that lead to sticky aggregations of cells that grow on solid surfaces — dental plaque is an example of a multi-species biofilm. When S. salivarius is grown on mitis salivarius agar plates the colonies take on a distinct gumdrop shape, which is blue due to the dye in the media (trypan blue) being taken up into the capsules around the bacteria. This helped us in species identification, since we had originally cultured the bacteria out of a stick of probiotic chewing gum.
"Not my gumdrop buttons!"
![[S.sal on mitis agar] 20140829_181448.jpg](https://steemitimages.com/DQmY8gAsK1LUFqFM2r6jhemYKP9YfCp2TsNddakAASQvTbD/%5BS.sal%20on%20mitis%20agar%5D%2020140829_181448.jpg)
S. salivarius is a prominent resident of the oral microbiome with high population numbers and numerous roles as a commensal organism and little cause for concern as a pathogen. S. salivarius subspecies are known to be part of healthy oral flora, and can produce small peptide compounds called bacteriocins that inhibit Streptococcus pyogenes (of "strep throat" infamy) and other bacteria such as Streptococcus mutans, which are involved in halitosis, plaque, and cavity formation [1]. Additionally, S. salivarius is armed with enzymes which can hydrolyse sugar linkages (α-1,6 glucans) formed in S. mutans biofilms; though this does not digest the difficult α-1,3 linked glucans that S. mutans forms in dental plaque. These general properties have formed the basis for Streptococcus salivarius being considered a suitable chassis for oral probiotic engineering [1].
Brush your teeth, folks...

[Source]: https://www.smbc-comics.com/comic/2013-02-26
Bacteria such as S. mutans forming biofilms and secreting acids that demineralize tooth enamel is bad news for human health, so a few colleagues and myself set out on a pet project to design an oral probiotic cavity-fighter with Streptococcus salivarius K12 as the foundation. I argued that given the existing high population numbers, ability to partially degrade biofilms, and antimicrobial activity from bacteriocins, why not give this organism a little bit extra to get the job done?
The gum we cultured S. salivarius K12 from, saving around $400. Costs, shipping time, and bio-security bureaucracy were an issue — the supermarket resolved all of these

Busting biofilms
Since S. salivarius could already digest α-1,6 glucans with an enzyme called a dextranase, we looked to arming it with another enzyme (mutanase) from Paenibacillus curdlanolyticus MP-1 to break apart the α-1,3 glucosidic linkages produced by S. mutans [2, 3]. The combinatorial approach of using both dextranase and mutanase together has been shown to inhibit formation of S. mutans biofilms more than the sum of the enzymes individual activities, but this was in vitro [3], and we needed a living whole-cell system where these enzymes were secreted in situ around dental plaque in the mouth. Thus, DNA encoding the P. curdlanolyticus MP-1 mutanase gene was fused to a S. salivarius secretion signal peptide to ensure the enzyme could make it outside the cell to chew away at plaque when expressed.
Since DNA is also a structural component in sticky biofilm matrices, we considered secreting the enzyme DNaseI to assist in crewing apart the DNA in tangled in dental plaque. After all, this strategy had shown success in engineering bacteria to become "seek and destroy" pathogen killers that bust into biofilms and release antibiotics [4], so it seemed reasonable to fold this into a preliminary design, despite that we never ended up getting the gene ordered and synthesized.
S. salivarius K12 on mitis agar and E. coli DH5α on LB + X-Gal plates
Bacteriocins: Peptide-based antimicrobial compounds
Breaking apart dental plaque alone is not enough to keep teeth healthy, so we thought to increase the existing arsenal of bacteriocins produced by S. salivarius such that specific targeting of S. mutans could occur. To date S. salivarius has already shown efficacy against oral and upper respiratory pathogens by secretion of antimicrobial peptides [1, 5], so allowing for direct killing of S. mutans seemed like the next logical step. To perform this task we investigated different publications for possible bacteriocins and lantibiotics, and arrived on Mutacin 1140 as the candidate for its ability to kill S. mutans [6, 7]. Interestingly enough in a stroke of tragic irony, Mutacin 1140 is actually derived from an isolate of S. mutans, JH1140 — life is cheap in the microbial world.
Bacteriocin structures of mutacin 1140 from S. mutans JH1140, and Nisin from Lactococcus lactis. Figure adapted from [8].
![Mutacin 1140 F1.large [cropped].jpg](https://steemitimages.com/DQme9kbze2hpxiLi8GXH3KPKr7jyZgmTor3raHgB4Ziwnfw/Mutacin%201140%20F1.large%20%5Bcropped%5D.jpg)
These bacteriocins have modified amino acids which allow for multiple intramolecular macrocycles to form, giving new functionality to the peptide. Mutacin 1140 appears to operate by forming pores and channels in the surface of Gram positive bacteria by rings A and B attaching to lipid II on the surface, then a facilitating a multimeric assembly of other mutacin 1140 molecules to form pores. While the exact mechanism is unknown this is a relatively conservative model.
The mature mutacin 1140 peptide does not form by itself, so, the export and post-translational modification system was also designed to be installed into S. salivarius K12 along with the membrane proteins allegedly responsible for immunity [6, 8]. The latter item (immunity) was important — no way in hell was I going to put in this much time into microbe engineering only to have the critter make something toxic to itself!
How do you prevent adhesion of S. mutans to teeth in the first place?
The principle of breaking up dental plaque and going for direct killing of cavity-causing microbes sounded great, but what about keeping bacteria like S. mutans from coming back? To this end we looked to a previous iGEM (international Genetically Engineered Machines) competition submission from 2008, where a 20 amino-acid long peptide dubbed "p1025", which was derived from a S. mutans adhesion protein, was used to competitively inhibit S. mutans binding to teeth [9, 10]. This peptide had shown efficacy in keeping S. mutans off of salivary receptors during in vitro assays [9, 10], so perhaps it would work in a mammalian mouth. Though, the intractable complexities of translating in vitro results to effective tools in multi-species living systems is not lost of me — this was an ambitious project to take on; especially for a hacked together team of undergraduates stumbling through the process.
Conclusions
This overall design would hypothetically make Streptococcus salivarius K12 a triple threat against tooth decay: A means to break apart plaque; the ability to kill primary causative agents of dental caries such as S. mutans; and a way to prevent S. mutans from re-colonizing tooth surfaces by competitively inhibiting binding. This all sounded fine and dandy, but issues with extremely limited research funding, a lab space eviction deadline, and other academic commitments, we had to abandon this project.
It was already ambitious to attempt engineering an oral probiotic from a difficult to transform organism, and even more so given I was spearheading this project in the 3rd/4th year of my undergraduate degree over the summer while taking classes and preparing for an incoming busy semester. Given the circumstances, and much to my dismay, the project was suspended indefinitely.
It is unsettling to have to kill your own brainchild, but I would love to reboot this project if I ever have the time, funding, and expertise to draw upon.
Thank you for your time.
References:
1.) P.A. Wescombe, et al. 2012. Developing oral probiotics from Streptococcus salivarius. Future Microbiol. 12: 1355-71.
2.) M. Pleszczyńska, et al. 2012. Gene cloning, expression, and characterization of mutanase from Paenibacillus curdlanolyticus MP-1. Protein Expr Purif. 1: 69-74.
3.) H. Tsumori, et al. 2012. Combination of Mutanase and Dextranase Effectively Suppressed Formation of Insoluble Glucan Biofilm by Cariogenic Streptococci. Interface Oral Health Sci. 215-217.
4.) I. Y. Hwang, et al. 2014. Reprogramming Microbes to Be Pathogen-Seeking Killers. ACS Synth Biol. 4: 228-237.
5.) M. Santagati, et al. 2012. Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. FEMS Immunol Med Microbiol, 65: 23–31.
6.) J. D. Hillman, et al. 1998. Genetic and biochemical analysis of mutacin 1140, a lantibiotic from Streptococcus mutans. Infect Immun. 6: 2743-9.
7.) S. Chen, et al. 2013. Site-Directed Mutations in the Lanthipeptide Mutacin 1140. Appl Environ Mirobiol. 13: 4015-4023.
8.) J. Escano, et al. 2015. Biosynthesis and transport of the lantibiotic mutacin 1140 produced by Streptococcus mutans. J Bacteriol 197: 1173–1184.
9.) 2008 MIT iGEM team Wiki page. URL: http://2008.igem.org/Team:MIT
10.) C. G. Kelly, et al. 1999. A synthetic peptide adhesion epitope as a novel antimicrobial agent. Nat Biotech. 17: 42-47.
Acknowledgements: I would like to thank Dr. Francis E. Nano for his guidance and assistance, especially with the purchasing of synthetic DNA.
@gktown showed me this and thanks for proving my point to @heroinehero that you CAN make one by posting basically your homework online with steem :D @dreamm too and @nepu
Absolutely fascinating to read about this, it seems like this approach to dental hygiene makes a ton of sense. I love your way of explaining everything - very easy to follow and engaging. Great post!
Much love - Carl
Glad you enjoyed this! I aim to keep generating content pertaining to science and biotechnology, but also trying to maintain accessibility to a wide range of readers.
Pleased to hear you agree that this seems like a rational approach for dental hygiene :)
This is the most interesting thing I've read today, thank you for sharing science in steemit.
You are more than welcome! It was a pleasure to be able to share this with this community and get such warm reception. More to come!
What a fascinating idea, but aren't we not more concerned with the genomic make up of our microbiome than what they represent at a species level? Last I heard the latest research indicated that while we previousely assumed that ecological niches within out gut were being filled by different species, that due to the horizontal transfer of genes and great variability within bacterial species that filling each niche wasn't so much a matter of species as of genomic regulation.
When we look at the bacteria above, is the activity mentioned always present? Or conversely can it be present in other bacteria which may fill their role easier?
Horizontal gene transfer is certainly a component of the microbiome, but without the appropriate organism as a chassis and the right genetic regulatory elements to express a gene it won't operate as expected. Indeed, the bacteriocins that are endogenous to S. salivarius are harbored on a transmissible megaplasmid of about ~190kbp that ensure it can spread to others of its own kind. However, those genes being transferred to other streptococci or oral bacteria may be much more difficult, as the recipient organism must be naturally competent, and the exogenous DNA must be intact.
The ecological niches that are occupied by bacteria are certainly still a major component, it is just now evident that there is more horizontal gene transfer than we had previously assumed; one does not negate the other, just expands the repertoire of usable (or unusable) genetic components.
I chose this as the chassis organism because of the high population numbers that already inhabit the average human mouth, and that it has existing probiotic functions in the oral microbiome. Perhaps other organisms could fill this role, but S. salivarius seemed like the most viable option if we were to achieve success as it already has existing activity which appears to be under constitutive expression; we just wanted to arm it further, but not so much that it burdens the cells or impairs replication/growth/function/life itself.
If you have any other questions I would be pleased to answer them! Hope this clarifies my line of reasoning and answers your inquiry :)
Very interesting !
Using probiotic for oral health sounds like a winning project, sorry to know there is no funds for it.
After seeing how well received this posting is, that might change!
Great post. In graduate school I did a major project on lantibiotic resistance mechanisms in gram positive bacteria so its great to see more reasons to study them. Sorry to hear you had to kill the project.
Very interesting project.
Could this probiotic gum, help with bacterial stones that form in the crypts of the tonisils? This bacteria is a major cause of tonsillitis.
Probably wouldn't hurt! S. salivarius is known to keep a few nasty critters at bay.
Great Post Mate. You Always Bring About Topics That Are Very fascinating To Read And Know About. YouR Approach To Dental Hygiene Is Very Sensible AnD Effective. thanks For Posting And Have A happY 2018 Mate!
I am wondering how those bacteria look like? They must be scary as monster.
Haha, hardly scary. Physically, they look like spheres only a couple microns in diameter that are linked together in twisted chains, like so:
We like to think we are in control of our microbes in/on our body, but we're just a bus. Happy new year :)
Hahah. We are the microbes’ planet.