Drug development (Pharmaceutics for Beginners, Vol. 1)
Pharmaceutics for beginners will be a series of blog posts where I’ll try to break down the huge topic “biopharmaceutics and drug delivery” into smaller articles that give a small insight in the topic and maybe can answer some of your questions or bring up even more questions, who knows.
Drug development
Let’s start with drug development the basic, and no, not the illegal stuff that Walter White cooks in his camper or you can buy from some suspicious looking people in the dark corners of the street. I’m talking about the stuff that you get when you are sick and need a prescription.
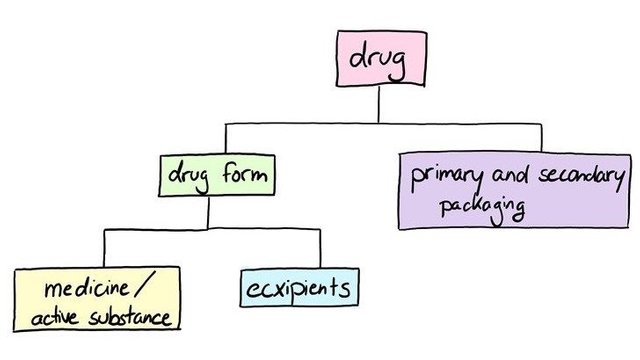
The so-called drug is more a generic term because the drug you get at the pharmacy is assembled and consists out of far more parts than only the tablet.
The box you’re handed in the pharmacy is the drug.
But when you open it ~probably on the wrong site with the leaflet facing you, but is this the wrong side? I don’t know~ you’re greeted by one of the secondary packaging parts. In fact, you’re already holding the other part of the secondary packaging which is the box. So, the secondary packaging parts are the box and the leaflet ~the leaflet you never read because folding it back in its original shape is impossible~. The primary packaging is the blister that stores each tablet separately or any other packaging that comes in direct contact with the drug form.
The drug form itself consists of two parts. The medicine or active substance and the excipient which can be cornstarch, lactose or any other excipient of a long list.
And that’s it. Those are the parts a drug consists of.
After we covered that let's move on. Have you an idea what the purpose of a drug form is? If not, let me tell you.
The drug form has different purposes like:
- vehicle function = carrying the active substance to the place where it has to do its job
- dosing = one pill contains exactly the calculated amount of the active substance it should have, take two means the double amount of active substance
- control of the drug effect = when the effect will kick in, how long the effect will last and how strong it will be
- stability of the active substance = prevents the premature onset of effect and provides a longer shelf life
- aesthetic aspects and product safety = make it look nice so you’re not hesitant to take it and it prevents overdosing
A lot of purposes for a small thing, right?
But before you can pick up the prescription it has to be developed.
From the first active substance synthesis to the first test in humans, it can take 3 to 6 years(!).
~if you want I can try to cover the development and discovery in another post because this would really blow up the concept of a small blog post~
The first test on humans is a clinical Phase I study (Phase I study), the probands take the drug while they’re monitored by professionals (which they are in any study). In this study, the drug form is tested on 20 to 80 probands and takes a couple of weeks. Because this is the first test on humans, this study is also called "First in Man" or FIM.
The main goal is to see how the pharmacokinetics and pharmacodynamics behave, also checking on the compatibility and the safety of the drug.
pharmacokinetics = the effect of the organism on a drug taken over time
pharmacodynamics = the type of drug action in the body, for example, the biochemical and physiological effects of the drug on the organism
After the Phase 1 study, the dosage gets adjusted and put into its final form. Like pills, syrup or injections.
Next, the drug must undergo two more clinical studies before the drug gets the regulatory approval.
The second clinical study (Phase II study) takes a couple of weeks to months and this time the drug is tested on a larger group of probands, around 50 to 200. The target of the second study is to check the concept of the medication and the therapy (proof of concept), finding the suitable therapy dosage (dosage finding) and in this study, there must be visible positive effects with the therapy.
In the third clinical study (Phase III study) the drug is tested on 200 up to 10.000 people and it takes months to years. The goal of this study is to have a significant evidence of effect (pivotal study) and the market release for the therapy.
After or during the third clinical study the drug gets the regulatory approval and is available in the pharmacies. Often after the market release, there is a fourth clinical study (Phase IV study) which takes years and needs 1000 to one million participants. The approval authorities often demand this kind of study to find rare side effects that are only visible in this huge group of participants. Commonly this study is also used for marketing purposes.
The whole clinical testing needs around 6 to 7 years.
And now we add the 3 to 6 years from the active substance synthesis and the 6 to 7 years from clinical testing phase we end up with a max of 13 years until a drug is finally available for purchase, now we subtract this number from the 20 years of the patent protections, which starts at the first synthesis of the active substance. You end up with 7 years where the pharmaceutical companies can make money to cover the development and research costs that are around 2,4 billion euro per new product.
After the 20 years of patent protection, the active substance isn’t protected anymore, and other companies are allowed to sell an exact copy of the drug with a different trade name.
The copy of a drug with no patent protection is called generics.
An example for generics is aspirin.
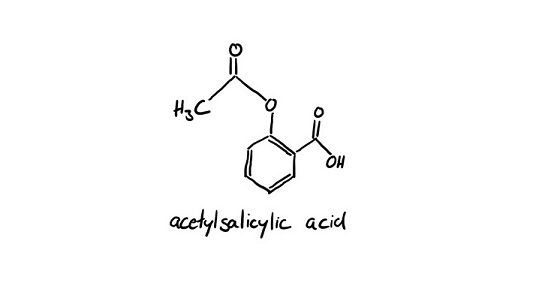
Bayer patented aspirin 1921 but due to the Versailles Treaty (1919) after the first world war, Bayer has been forced to give up its rights in the territory of the victorious allies the US, France, and Great Britain. In the US the pharmaceutical company Sterling Drug bought the market rights. In 1994 Bayer AG bought Sterling Drug from its temporary owner, Kodak, for 1 billion USD, and since then, Bayer has the name rights on aspirin back.
So, aspirin is the original drug and the generics to aspirin is for example ASS-ratiopharm. The generics contain the same active substance from aspirin which is acetylsalicylic acid and the same excipients cellulose powder and cornstarch.
TIPP: If you want to save money on prescription free drugs like painkillers look up the ingredients in the name brand and check if there is a brand that uses the exact same ingredients. Often the generics are much cheaper than the original.
Thank you for reading, if you have any questions feel free to leave them in the comment section
(ノ◕ヮ◕)ノ*:・゚✧ Next on Pharmaceutics for Beginners Vol. 2: biological barriers
References:
Picture by qimono on pixabay.com
Lecture "Arzneimittelentwicklung" by Dr. Eva-Maria Collnot
Warum die Entwicklungskosten zunehmen
Acetylsalicylsäure - Geschichte
This is an awesome write up for a newbie (I presume from your profile). I guess suesa did some tutoring beforehand.
Your article is an interesting one for me, considering the fact that I have once worked as a pharmacy technician for some months.
Lemme start with your definition of of pharmacokinetics:
Perhaps you mean the other way round. In this case, it should be the effects of the drug taken on the organism over time and not vice versa.
Then I have a question: what are probands? Who qualifies/volunteers/get selected as probands? What if the drug has deleterious side effects on these humans?
Yeah, could be possible that there is a tiny mishap. Thank you for letting me know :)
Probands are the people that are volunteering to try those new drugs after they passed the animal test.
Everyone is qualified as long they are in an overall good health constitution and match specific criteria depending on the drug that they're going to take.
Often the drugs are tested only on men to avoid any damage on the female ovum to lower the risk of permanent damage. The sperm is always "freshly" produced, while the female ova develop when the person is still an embryo in the mother's womb. This is just to make sure that the offspring of the probands don't suffer any damage from the study.
And if there is the tiniest sign of a deleterious side effect, the study will be immediately stopped and the active substance gets improved or dismissed.
Sounds fair enough. There must be an element of motivation for people to voluntarily donate their bodies for such experiment, perhaps some financial rewards?
That is indeed a good introduction, definitions, and history. Thank you!
Looking forward to upcoming parts :)
Hi, thanks for this comprehensive overview, ut's a nice and interesting write-up.
I saw that you used german sources... May I introduce you to the @de-stem project, the german-language sub-community of steemstem...Maybe it's interesting for you ;-)
You're welcome :)
sure I'll look into the German steemstem and maybe publish some articles in German :)
Tu dir keinen Zwang an ;-)
es wäre leichter in deutsch, da kann ich mir das übersetzen sparen ^^"
Ich schau einfach mal was sich so ergibt :D
auf Englisch hast dafür halt das größere Publikum. Bei uns fahren daher auch viele zweisprachig und posten mal so, mal so. Unseren tag hab ich noch nicht erwähnt: #de-stem.
Einen guten introduceyourself-post würd ich dir auch dringlich anraten ;-)
Vorschläge was man so rein schreiben könnte? ;)
Was dich hierher verschlagen hast, wer du so bist, was du machst, wohin dich dein Weg führt, welche Farbe dein Krafttier hat...das Übliche eben :)
in dem Zusammenhang vll. interessant:
https://steemit.com/community/@theaustrianguy/let-s-make-introduceyourself-great-again-an-update-on-welcoming
Being A SteemStem Member
woot woot!
And this, kids, is why developing homeopathic "medication" is so much more profitable. No tests, no annoying regulations, just easy money. I'm definitively in the wrong industry.
and all those people that are trying to cure cancer with homeopathic "medication" ^^"
I'm not against homeopathic, but this stuff seriously can't cure cancer.
Congratulations @msfrozendust! You have completed the following achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
SteemitBoard World Cup Contest - Russia vs Croatia
Participate in the SteemitBoard World Cup Contest!
Collect World Cup badges and win free SBD
Support the Gold Sponsors of the contest: @good-karma and @lukestokes
Congratulations @msfrozendust! You have received a personal award!
Click on the badge to view your Board of Honor.
Do not miss the last post from @steemitboard:
Congratulations @msfrozendust! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Do not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!