Chiral porphyrin hosts as detecting agents for polluted water: The introduction and background knowledge.
AUTHORS NOTE:
Two times in my life I was fortunate enough to work with a research group exploring the applications of porphyrins; once in America, where we synthesized chiral porphyrins for their applications as selective anionic receptors, and once in Austria where we synthesized dye-functionalized polymers by ROMP using either porphyrins or perylenes for triplet-triplet annihilation. In-between these two endeavors, I began to form an idea for an application of the chiral porphyrin hosts; my hope here is to present said idea for all, including my peers, superiors, and those with a budding interest in chemistry, in order to receive feedback. I desire to be a research scientist one day in the near future (I'm currently waiting to attend graduate school) and, if this idea proves fruitful, would greatly enjoy exploring it further. Please pull no punches if you do decide to provide feedback- if it's been done before, or disproven, or would be unnecessary due to superior methods already existing, I need to know; I don't have the kind of access to current research that I once did, so these are things that might have slipped my eye. Two final caveats- though I aim to present this in the most scientifically sound way possible, it's not an article, so while I'll cite when I feel it's necessary, the standard met will likely not be ACS; additionally, I feel the tone will deviate from such a standard, and I'll also take the time to explain things that might not be considered necessary for a publication, mainly for the benefit of those not yet as versed in the field as I. References provided haphazardly because I'm a haphazard cunt.
INTRODUCTION
Porphyrins possess a specific set of qualities that are often invaluable in host-guest chemistry (a field of chemistry in which two molecules are bound to each other by forces other than covalent bonds; typically, shape, chirality, hydrogen bonding, and dispersion forces are the deciding factors for the success of these projects.), including their shape and size along with the ability to craft host complexes with chiral interiors as well as their UV properties. Utilizing porphyrins to synthesize host complexes is an idea that's been explored for over 30 years and, due to the success of these endeavors along with the reliability of the process, is still a popular field in contemporary supra-molecular chemistry; indeed, a littany of porphyrin hosts have already been synthesized, and while a myriad of applications for these compounds have been explored, the nature of all science is that we never stop asking questions. My question was this: could we utilize these compounds, or perhaps synthesize specific and targeted compounds, to detect pharmaceutical and other agents in natural water sources?
It might be a long shot- there are already existing methods for specific compounds, however there are always more things to find. Though I mainly focus on pharmaceuticals specifically, any hazardous contaminant that could be found would prove a useful result. There were a few reasons for my specific question. First, the idea goes hand in hand with the entire prophyrin host-guest process; the general outline of such a procedure, or at least the one I hope to employ, is that the ability of our host complex to selectively bind to a specific molecule an a solution containing multiple compounds (and often an enantiomeric solution) is measured, typically by UV spectroscopy. A great bulk of this process could carry over to our new application. Additionally, measuring and detecting the presence of pharmaceutical agents in natural water bodies does not have a catch all method- though specific compounds can be detected, frequently by employing high performance liquid chromatography (HPLC), there's no catch all method; specific compounds have to be measured by specific methods, and there are far far more compounds that we don't yet have the ability to measure than compounds we do. In that light, if successful, porphyrin hosts could be used to measure new compounds and help to expand the list of pharmaceuticals that we can detect.
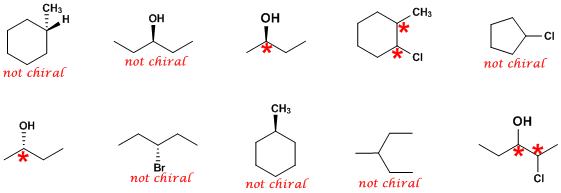
Chirality is often referred to as handedness; a chiral compound is a compound with a unique shape that is non superimposable on its mirror image. We refer to that as handedness because that foreign sounding explanation is greatly simplified by looking at your hands. Holding your hands spread evenly, thumb to thumb, it's easy to see that your left and right hand are mirror images of each other, yet it's impossible to face both hands in the same direction while retaining this mirrored quality. This is what is known as imposing; any compound that has a mirror image thats non-superimposable on it is chiral, and the pair are said to be enantiomers of each other. A chiral center is any center (almost always an atom) in a molecule that has 4 (very rarely deviated from, but not relevant to this paper) unique groups bonded to it. Failing this, a compound is not chiral, also known as achiral.
Some interesting things come from this- when a porpyrin is attached to a chiral compound, it can form 3-dimensional structures that actually have chiral interiors. This chiral interior can sometimes encapsualate specific compounds much more readily and effectively than it can said compounds enantiomer; this is often referred to as a lock and key relation, and that fits well (no pun intended). I prefer to think of it as a foam ball- if you were to carve out part of the interior of a foam ball, the only thing that would fit in the now hollow portion would be the original removed portion or anything that was an exact duplicate to it, due to the unique shape present. This is one of the driving forces behind porphyrin host; along with other intra and intermolecular forces, the unique shape present is critical to the ability of the host to bind to a specific molecule.
The ability to sufficiently measure the ability of a porphyrin host to bind to the target molecule is made easier by the reliable UV-vis qualities of porphyrins. UV-vis spectroscopy is a form of instrumentation in which light in the UV and visible range (a refresher, the UV range is 10 to 400 nanometers and the visible range is ~400-700 nm; in spectroscopy, countless individual waves of light matching every wavelength in this spectrum are used) is passed through a compound; as this happens, light that matches the exact wavelength of an energy gap in the measured compound can be absorbed, which causes the absorbing electron to go up an energy level. As some light was absorbed, the remaining light will, when measured, have a lower intensity for the absorbed wavelength. From this, it's actually possible to calculate the concentration of the absorbing species, as the intensity of the initial wavelengths are already known. The poster presented at the beginning of this paper expands upon this; the important fact is that because of this, we can accurately measure the concentration of compounds with this method.
A few more nuances of UV-vis spec are worth discussing. The general procedure is as follows: first, free host is measured in order to determine its absorbance pattern. Following this, gradually increasing volumes of a stock solution of guest are added. The reason for this two part process is that absorbance is a spectrum, and the spectrum of unbinded host almost always overlaps with host guest complex. Because of this, we first measure free host in order to subtract those measurements from the host-guest spectra so as to not falsely magnify the results. This can, in fact, be quite dire, because the concentration of free host will be several orders of magnitude higher than the host-guest complex; one might assume that as the concentration of free host goes down with every titration, subtracting the same amount from every measurement even though the concentration is constantly decreasing is inaccurate. Technically this is actually correct, however due to, again, the drastic difference in concentration of free host vs host-guest, this is a negligible difference. The free quest as well has it's own peaks, however said peaks are frequently far off of the host guest or host peak, and the peaks themselves are very small if using an applicable porphyrin.
With the synthesis of these compounds having already been documented by the poster (deviations might occur, but they're as of yet undocumented), it is now possible to move forward with my own idea. It's straightforward, first design a porphyrin host that selectively binds to a pharmaceutical agent of interest. Special care will have to be taken to ensure that, if it binds to any common component of natural bodies of water, the spectra is known and controllable. Should solid components be present in field samples it's possible they might need to be filtered, depending on the solubility of the target molecule in them. The main hurdle is ensuring that the spectra of the natural samples is able to be controlled for- there are innumerable components in natural water bodies, and to ignore this could likely present an indecipherable readout. A possible idea is to, utilizing other analytical methods, determine the average components of a random sample. Following this, we synthesize such a sample in house, take a reading, and then add guest in order to get a read of how on site samples might behave. Hopefully, if sufficient hosts are created, we can streamline this whole process.
For the most part this is uncharted territory- I can imagine that several changes might be necessary, which is another reason I wanted to put this idea out for everyone to see. Please pull no punches, I need to know. Thank you!
References: