The Elements
In Evolution of the Atomic Model 1 and 2, we saw how scientists came to understand how atoms are structured, and how our model of them developed. Here is a reminder of the masses and charges of protons, neutrons, and electrons.
Atoms are all made of these particles. But there are many different kinds of atoms, which all behave differently. These are called elements.
An element contains just one kind of atom.
There are more than 100 known elements. About 90 of them are found in nature. The rest have been made in the laboratory. These man made elements tend to be very unstable, so they decay into other elements very quickly.
To make life easy, scientists use symbols for the elements as a sort of shorthand. Sometimes the symbol for an element seems counter-intuitive, this is because the original name for the element came from Latin or Greek.
The Periodic Table
The data that we know about the elements is displayed on the Periodic Table. The version of the table below tells us the name, the symbol, and the number of protons in atoms of each element. Every atom of the same element will always have the same number of protons. The number of protons is called the atomic number.
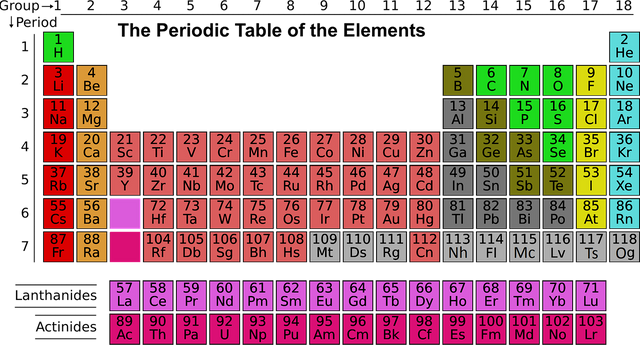
You'll notice that the table is arranged into columns and rows, called groups and periods. Elements in the same column of group are likely to have similar properties and behaviours. We'll learn more about this when we look at electronic structure.
For now it is enough to know that an atom with a particular number of positively charged protons will have the same number of negatively charged electrons, bringing the overall charge of the atom to zero. the neutrons do not carry charge, so they don't affect the atom's overall charge.
But neutrons do have mass, so they will affect the overall mass of the atom. So symbols for the elements will often be written as follows:
Some Examples of Elements

Well done! This post has received a 13.51 % upvote from @litasio thanks to: @revisechemistry. Whoop!
Ads: Mining Burstcoin with Hard Drives!
Congratulations @revisechemistry! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!