The Chemistry of Fireworks (Happy Fourth of July!)

(image from here)
Specifically, I’ll be focusing on fireworks known as “aerial shells,” which are the ones that you see in the sky during 4th of July events.
There are multiple components making up an aerial shell, let’s start off by describing succinctly what each one does
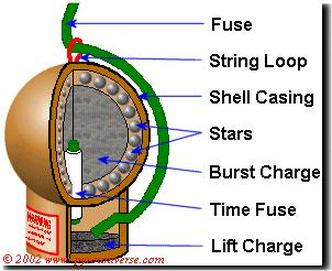
(image from pyrouniverse.com)
Fuse - Does exactly what it sounds like. You light this part and then run away.
Shell Casing - Very simply a case for it all, taped together with a sturdy wood tape.
Stars - These are what shine bright in the sky. They are made of a mix of chemicals, described below.
Burst Charge - This is a bunch of gunpowder in the center that will cause the actual burst
Time Fuse - A fuse inside the aerial shell which is made so that it will finally reach the burst charge when the aerial shell is at its apex, or the peak of its ascent into the sky
Lift Charge - The shell is put into a mortar, so when the fuse hits this part, the actual shell itself will go flying up into the air
The stars are where the real magic happens. You can do a few neat things with these besides just pack them around for a big burst.
For example, if they're arranged into a pattern, the firework can make shapes like smiley faces and stars. Yep, it's really that simple how they make the shapes. The stars are just lined up on one side of the shell in that shape, so that when it bursts (from the center, the "burst charge"), they will fly out in one direction and form that shape in the sky.
Another thing that can be done is putting smaller aerial shells into a large one. This is what creates fireworks that burst in two parts - the ones that have an initial big bang and then all around that there are a lot more, smaller explosions to follow.
Quite a lot of these components are made out of obvious stuff, like the burst charge and lift charge being filled with gunpowder. But what are these stars made out of? Well lucky for anyone who wants to make aerial shells at home, you can find the formulas for these stars online very easily.
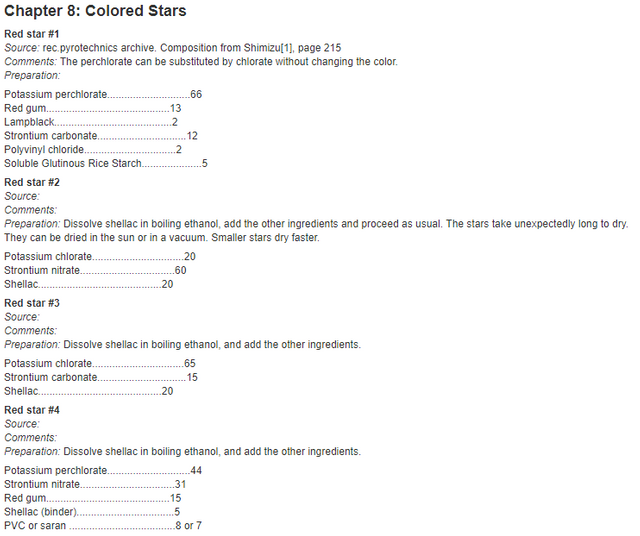
Generally speaking, there are the chemicals that give the stars their texture and ability to burn brightly for a while (dextrin, rice starch, mealpowder, shellac, charcoal) and then there are the things that give them colors and strobe effects (chlorates, perchlorates, nitrates, sulfur, parlon). More information is here.
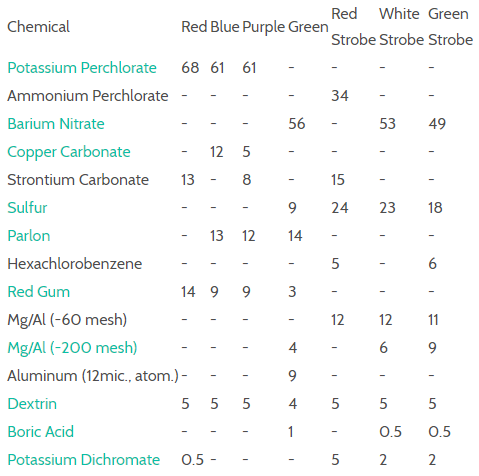
Let's focus on the perchlorates. Now, while I don't recommend you make these at home, in case you try anyway, I strongly recommend two things: You will probably try boiling bleach to make the perchlorates, be sure to do this in a well ventilated area, preferably outside. Also, do not handle perchlorates in your kitchen as they are highly reactive with sugar. Another thing as general advice if you try making aerial shells - do it outside in an open area and wear plain cotton clothes.
Why are the perchlorates so important? Well, they're basically just "high quality" compared against nitrates - they shine brighter, more colorfully, and are a little harder to create.
The color of each perchlorate is determined by the metal that is bonded to it. Perchlorate alone is an ion (an anion, specifically, which is a negatively charged ion), which looks like this
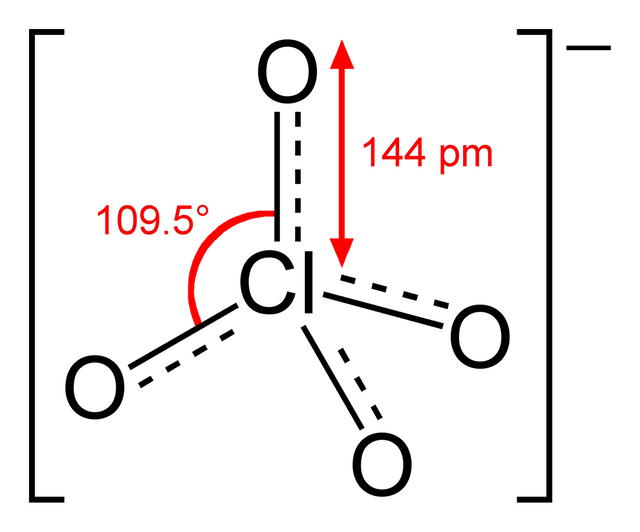
(image from Wikipedia)
If say, a barium cation (a positively charged ion) were to bond to it, like so
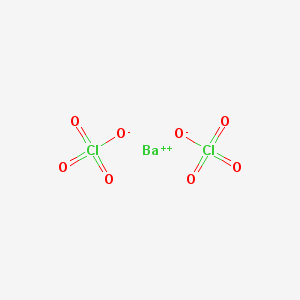
(image from here)
and you incorporated that into your firework's stars, then you would have stars that shine green! This is because the color is determined by this cation.
Here is a graphic which summarizes all of this very nicely:
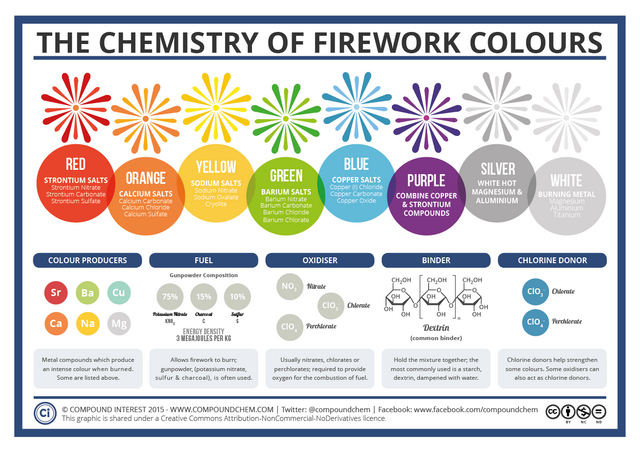
(image from CompoundChem)
If these color matchups seem familiar at all to you, that might be because they are the same for flame tests (which is a common high school experiment)

(image from CompoundChem)
The only difference here being copper, but that is due to the different oxidation states - Copper (I) vs Copper (II). That just means one is a copper cation with a charge of +1 while the other is a copper cation with a charge of +2.
These colors are simply intrinsic to these metals, when they emit photons they will emit them at a given wavelength for that element.
That just about sums it up. Hope you all have a fun Fourth of July, even if you're not American! ;)
Happy Fourth of July :)

I see that none of your images are credited and probably most of it copyrighted. Try to find CC images in their place.
It's important to also list your sources when writing stem content.
Ah okay, sorry about that! Will edit it soon
edit: ok done!
Happy 4th of July! God bless America!
Bachuslib is right, you'd need image sources on the platform. It's pretty easy to do, simply copy the link to the website you got the images from and then paste it in the smaller bracket of this markdown code.
[write 'image source' here](paste weblink here)Notice how the brackets are close to each other without spacing?My friend Kat did a very explanatry post on this: Markdown guide
Remember, the images have to be free from copyright. you can find them on pixabay.com, pexels.com and the likes.
Need help? you can reach steemSTEM on discord here: https://discord.gg/Eu8JjW

upvote for me please? https://steemit.com/news/@bible.com/2sysip