Let's Learn Anatomy!!! #4 - Amino Acids and Proteins
Okay, it's January 2nd and you have finally recovered from the previous night's binge drinking and embarrassing attempt at Irish folk dancing. You've decided that your New Years Resolution will be to lose 15 lbs, and by God, you're going to stick to it this year and get the body you've always wanted!!

Credit: http://www.kingofthegym.com/56-yo-lifting-weights-and-trying-to-lose-fat-weight-lifting-q-and-a/
You go to your local book store, grab the most popular dietary book they have, and take a seat. As you begin to thumb through the first few pages, you notice a couple of things:
- Finely chiseled abs start to look somewhat creepy the more you stare at them
- Apparently you should be "counting your macros"
You slowly close the book, lean back in your chair and begin to ponder... what on earth are macros?
Let's find out!!!
It's Time to Build Us a House
Macromolecules, or macros, are basically what you're built out of. They're considered organic compounds, seeing as that they all contain a considerable amount of carbon atoms.
It should be noted that simply having carbon atoms in the molecular structure doesn't make the molecule "organic".
Unfortunately, a solid distinction between organic and inorganic compounds doesn't exist. In fact, you'll most likely get a different definition depending on whether you ask a chemist or biologist to define them.
Now, to try and gain some perspective on how important macromolecules are, it might be useful if we think of a human cell as a house.

Hmmmm... Preferably one with more than just two people building it...
Anyhow, in this example, macromolecules would be the foundation, frame, insulation, roof, doors, windows, and wiring. Basically, everything that makes it a house.
Now, the things that make the house not only look good, but also livable, like paint, furniture, and electricity would be various things like minerals and other inorganic compounds (this isn't a perfect analogy, but it can serve our purposes for the time being). I mean, just think to how useless the wiring would be if there wasn't an electrical current running through it.
Inorganic compounds can generally be thought as not containing carbon atoms. The frustrating thing is, it's not always true. For instance, carbon dioxide (CO2) obviously contains carbon, yet it's considered an inorganic compound. If it were up to me, chemists and biologists would be locked in a room until they decide on a universally accepted definition. Until that happens, it might be best to think of inorganic compounds as simply compounds that aren't organic.
I know... its profound stuff.
Just so that we're all on the same page, here's a quick look at a few examples of each type of compound.
| Organic Compounds | Inorganic Compounds |
|---|---|
| Proteins | Water (H2O) |
| Fatty Acids | Carbon Dioxide (CO2) |
| Carbohydrates | Carbon Monoxide (CO) |
| Vitamins | Minerals |
| Neurotransmitters | Ammonia |
This only scratches the surface when it comes to how many different compounds are out there, but it'll do for the time being.
Now, if we're going to build a useful house, the first thing we need to do is lay the foundation and construct our framing. To do that, let's dive into the structurally sound world of the protein.
Protein... More Than Just an Awful Tasting Vanilla Shake
Have you ever wondered why gym nuts are always talking about protein? I mean, what exactly is so useful about consuming copious amounts after a workout?
For gym nuts, it all comes down to building muscle. We've all heard that consuming animal protein in the form of meat is a great for our muscles, so maybe that's a good place to start.
Let's eat some chicken!

If you recall our last blog post, Basics of Biochemistry, we discussed an enzyme in our saliva that catabolized the sugar in bread as we ate our turkey sandwich. This time however, nothing in our saliva packs a big enough punch to begin the digestion process for protein. However chewing is extremely useful, and performs the function of mechanically shredding the protein and increasing the surface area that will be exposed to the powerful digestive enzymes farther down the line.
Upon entering the stomach, the chicken is met with the immensely strong hydrochloric acid we met last time. The acid begins to denature the protein, or essentially break it down to a more simple form, called its primary structure. You see, proteins come in all shapes and sizes. In fact, proteins can actually fold on themselves, creating very unique, and as we'll see in a bit, essential shapes that define its function.
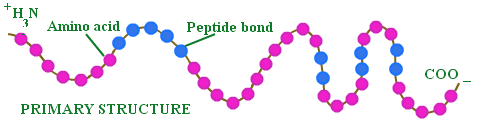
Credit: http://chemistry.tutorvista.com/biochemistry/structure-of-proteins.html
The thing is, these shapes can make it extremely difficult (if not impossible) for the body to absorb the protein. Obviously this is problematic, so the stomach acid acts to unfold the protein into a simpler form that will then be further broken down and more readily absorbed through the intestinal lining.
Amino Acids! The Building Blocks of... Building Blocks?
Hydrochloric acid is an extremely powerful acid, yet it's not enough to fully catabolize the protein we consumed. The stomach also produces an enzyme called pepsinogen, which becomes activated when it encounters the hydrocholric acid, and is now called pepsin. Pepsin is what we call a protease, which is a digestive enzyme that specializes in breaking apart proteins into their most basic structure, an amino acid.
Quick side note: The cola beverage Pepsi was originally marketed as a digestive aid. Rumors have claimed that pepsin was an active ingredient in the original recipe, but in reality it never was. Instead it's named after the Latin word pepsia, which simply means digestion.
Once our partially digested soup (chyme) is ready to move into the small intestine, an extremely powerful valve at the base of the stomach, called the pyloric sphincter, opens up and releases the stomach contents into the intestinal tract. This is where more digestive enzymes act upon the protein, finally completing the process of ripping them apart into amino acids.
There are over 500 different amino acids that we know of, yet the body only uses 20 or so of them. We divide these 20 amino acids into two separate groups: essential and nonessential
9 of the 20 amino acids are called essential because the human body is incapable of synthesizing them on its own, yet requires them to function properly. The other 11 amino acids are called nonessential, and while still just as necessary the essential amino acids, the body can synthesize them on its own, meaning you don't need dietary protein to make them.
Whether the amino acids are essential or nonessential, they will be used to build the vastly immense human proteome, which is the entirety of the proteins expressed in the human organism. Now the proteome isn't fully complete, but current estimates put it at somewhere around 30,000 different proteins for a human being.
Just think about that... From just 20 amino acids, your body can construct 30,000 different protein combinations!
So What's the Big Deal? What Are Proteins Used For?
What you need to understand about amino acids is that they can be hydrophilic, meaning that it bonds with water, or hydrophobic, meaning that it repels water.
When you want to build a protein, you'll place the various amino acids into a distinct pattern by bonding them to one another, creating an amino acid chain. When you have 2 amino acids linked to one another, we now call it a peptide. If we have 10 more amino acids, we have what's called a polypeptide. If we get 50 or more amino acids linked up, we now have what's called a protein.
Peptides, polypeptides, and proteins all have their own unique functions. At a later date I fully plan on discussing the various functions of the peptides and polypeptides, but for now let's just stick with protein.
Depending on which amino acids are used to construct the protein, you'll have a pattern that is littered with hydrophilic and hydrophobic aspects. These differing properties cause what is known as protein folding.
To better visualize this, think to magnets. If I gathered a bunch of magnets together and threw them into a container, the opposing ends would cause certain magnets to attach to one another, and others to repel.
You would see different patterns and shapes of magnets.
This same thing happens to proteins, and it's name and function depend on which shape it takes. There are 4 basic structures a protein can take. These are the primary structure, secondary structure, tertiary structure, and quaternary structure.
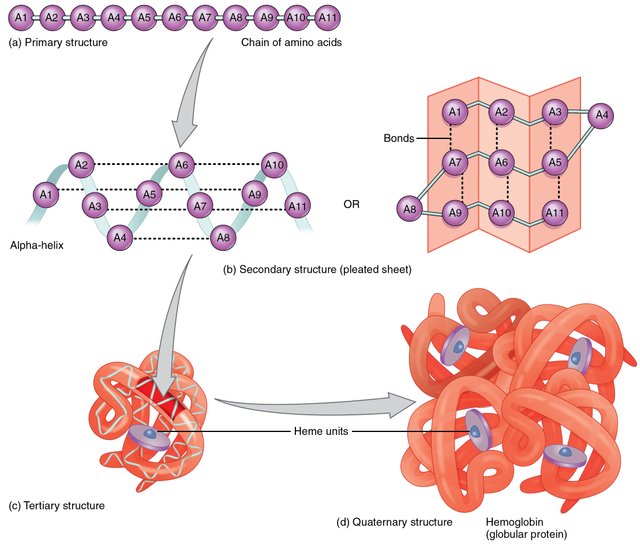
Credit: http://baltichost.tk/gevo/shape-of-proteins-liji.php
The important thing to realize here is that proteins can vary dramatically from one to another, depending upon the folding pattern it takes. It's this complicated folding process that creates the vast proteome we discussed earlier.
What About That Chicken Dinner?
Remember that the last time we saw our chicken dinner, it had just been bathed in acid and was being released into the small intestine. Once the amino acids have been absorbed into the blood stream, they can now be shuttled around the body and utilized by whichever cell needs them.
For instance, let's say that a woman is going into labor.
A structure in her brain called the pituitary gland will begin to secrete a hormone called oxytocin, which causes the uterus to contract powerfully, creating the lovely labor pains every woman enjoys so much.
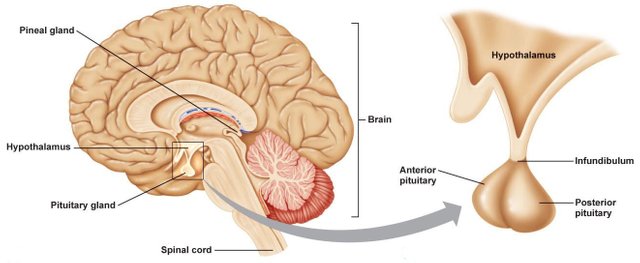
Credit: http://www.lifeharmonizer.name/index.php?id=698
In order to continuously produce the hormone necessary for the birthing process, the pituitary gland is going to need to create lots of oxytocin. To accomplish this, amino acids will be scooped up by the pituitary and linked together with peptide bonds to create oxytocin. The gland will continuously produce more of the hormone, causing the contractions to get closer and closer together, and more and more powerful.
She may not know it, but if it wasn't for the meals she had eaten throughout the past few days, she wouldn't have the available amino acids to help with this process. Granted, the body does have ways around this (the body will steal proteins from muscles), but we'll discuss that in a later post.
The big takeaway here is that the protein you consume through diet is reused in the body, in whatever capacity the body desires. Depending on which cell the amino acid is utilized by, those amino acids can become hormones, enzymes, or even muscle protein.
Yes, that's correct. The reason that gym nuts are eating so much protein after a workout is because their muscles quite literally experienced a traumatic injury.
When you work out, the stress of the exercise causes what are called microtears in the muscle. Now, it's not enough to truly and profoundly injure the muscle tissue, but it is enough to signal to the muscle that it wasn't strong enough to complete the task at hand.

Credit: https://www.studyblue.com/notes/note/n/ap-lab-test-2/deck/6213698
The muscle is smart, and decides that in order to prevent a true injury in the future, it's going to become stronger so that it can handle the load more efficiently next time. Amino acids are brought to the muscle cells, and the contractile proteins myosin and actin are synthesized, making the muscle more powerful.
Protein accounts for roughly 20% of the mass in a typical human being. Protein has a plethora of functions in the human body, including, but not limited to:
- forming the structural cytoskeleton of many different types of cells
- interacting with receptors on the surface of each cell causing a very specific reaction to occur
- connecting every single tissue in the body to one another
Despite all this, protein is only one type of macromolecule. Next time, we'll meet another member of the macromolecule family, the lipid, and finally figure out what the deal is with fat.
See ya next time!!
In case you missed the earlier posts in the series:
Let's Learn Anatomy #1
Let's Learn Anatomy #2
Let's Learn Anatomy #3
Here are a few other posts that might interest you:
What Is a Heart Attack?
Why Are Human Babies So Incredibly Weak???
Why Is Your Butt So Big???
Evolution, Society, and Cryptocurrency... How Does It All Relate???

Really great stuff, I am a medical student so would appreciate and advice and feedback you have on some of my blog posts! I really enjoyed your description of macromolecules and amino acids. Also the examples you give really make the post easy to follow and very understandable!
Thanks! I'm happy to help in any way I can.
Congratulations @justincottle! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP