What Happens When You Drink Seawater?
In a typical adult male, 60% of the body weight is composed of water (for example a man of average weight 'about 70 kg', body water weighs approximately 42 kg "42 liters'). Total body water (TBW) is divided into 2 two major compartments:
40% in the intracellular compartment
20% in the extracellular compartment: about 15% in the interstitial compartment and only 5% in the plasma compartment (= blood).
So as you can see, water is the most important liquid in the body and its quantity is regulated by precise mechanisms.

To answer the question above, I'm going to remind you of some of the most useful physical and physiological principles.
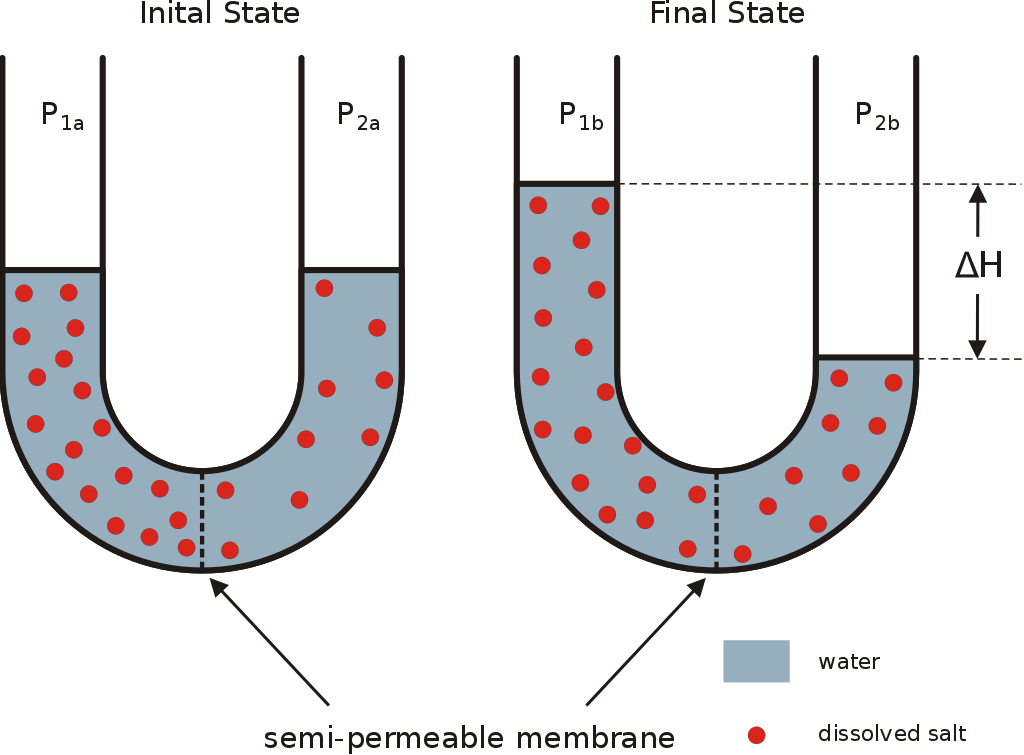
If you take two different adjacent media with the same volume of water but with different concentrations of the same solute (sodium ion for example) separated by a fully permeable membrane (the sodium ion and water can easily and passively diffuse between the two media). After a while, you will notice that the sodium ions passed from the most concentrated medium (hypertonic) to the least concentrated medium (hypotonic) until the equilibrium of the concentrations (a state of balance). This law is ubiquitous (found everywhere) and valid for all the different liquids and gases without exception. it is mainly due to the anarchic thermal motions of the particles, which is why the equilibrium point takes a while to be "achieved". Now let's take the same example but this time with a semi-permeable membrane allowing only water to pass. What will happen?
The sodium ions create a pressure gradient between the two media (pressure difference), water also moves from the hypotonic medium to the hypertonic medium according to a differential pressure calculated by this formula:
P.Hyd1 - the hydrostatic pressure of the medium 1
P.Hyd2 - the hydrostatic pressure of medium 2
P.Na1 - the sodium pressure of medium 1 called oncotic pressure
P.Na2 - the sodium pressure of medium 2
This is the law of osmosis which is applicable in the case of a semi-permeable membrane as shown in the image below:
The osmotically active particles are by definition particles which can not cross the semipermeable membrane and thus create an osmotic gradient calculated in osmoles, and they are limited to generating the osmotic motion. The concentration of the osmotically active particles is called the osmolarity in calculated in mOsm/L
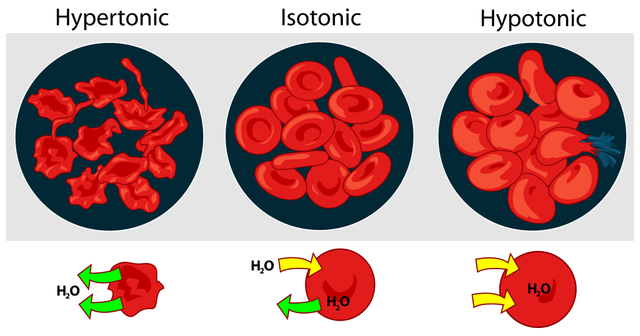
The cell membrane has a sort of semi-permeable membrane, so there is a permanent movement of water between the intracellular and extracellular medium. (for instance, red blood cells as shown below)
The main osmotically active particles are sodium, potassium, urea, and glucose. The osmolarity of the plasma is calculated according to the concentrations of these particles using this formula:
Osmolarity = 2 (Na + K) + Glucose + Urea
it's equal to almost 300 mOsm/L
Now I hope you understand enough about the physiology of water exchange in the body.
So what happens if you drink sea water?
First of all, you should know that seawater is very rich in salts especially sodium and has an osmolarity between 1000 and 1100 mOsm/L. When it enters the body, as its salt compounds are osmotically active, the plasma osmolarity increases significantly usually creating a hypernatremia and a call of Water is established from the least concentrated medium, AKA the intracellular medium to the extracellular medium according to the osmosis law. The result is intracellular dehydration and extracellular hyperhydration.Symptoms vary a lot according to the quantity of water ingested. The intracellular dehydration is marked by an intense thirst which is constant, sometimes a mild fever, drowsiness, mental confusion and coma in extreme cases, more rarely seizures.
On the other hand, Hyperhydration is marked by a rise in blood pressure, headaches, and abundant urine.It may take some time for these symptoms, therefore, the body must eliminate excess salts and liquid immediately , the problem is that kidneys have a limit to concentrating the urine.That being said each amount of particles requires a minimum volume of water for their evacuation.
This results in a greater loss of water than the amount that has already been ingested and global dehydration installs. So even if you drink seawater to cover the lack of fluids in your body, you will end up aggravating your initial dehydration.
References :
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1304728/
https://www.ncbi.nlm.nih.gov/books/NBK21739/
Picture Credit:
Thumbnail - pixabay.com
All the rest- wikimedia.org
One more thing, Join the #steemSTEM channel, A community project to promote science technology engineering and mathematics postings on Steemit.
It's so bitter to drink.
Yeah it seems like that