Crude Oil, Part 1
CRUDE OIL

Crude Oil
In our ever changing civilization, we must be thankful for all of the vast expansion that is taking place. We are able to finish tasks that took our ancestors eons to do, in a matter of months. We are able to travel half way around the world in just a matter of hours. We will soon be able to travel to the vast beyond in the years to come. But, what do all these factors have in common?? A source. In our everyday lives, we use a form of energy that is trapped within another form of matter to go along with our everyday lives. We drive to our destination, we recieve goods transported to us, and most impotantly : We are always moving. In this post, I am going to be explaining one of these sources, and one of the most important sources in the petroleum industry, Crude Oil.
In any oil bearing formations, such as that for crude oils, the fluid present in the constitution are in order of increasing densities with the presence of defined boundries. With any boundary conditions, there is an upper as well as a lower boundary. In the process of oil production, the upper boundary is of great importance to us, as this boundary has the most concentrated layer of gases. When I refer to the term boundary, we should imagine fluid that is within a pipe. If we introduce the cartesian co-ordinate system, whereby a pipe is running horizontally, we define X=0 at the base of the pipe, as well as X=x being at the maximum distance away from the base of the pipe, within the pipe. In most cases, the pipe used is that of a cylindrical shape, therefore the maximum distance away from the base of the cylinder would be it's diameter (this is an example that I am using to explain the different layers of crude oil, if we assume it is flowing in a perfectly cylindrical shape, such as a pipe) . At X=x, the concentrated fluids consist of free gases, which is also referred to as gas cap in the crude oil industry. Within this layer, the most abundantly found component of gases such as methane , carbon dioxide, diatomic nitrogen as well as hydrogen, along with other high molecular weight compounds (mostly larger hydrocarbons) present in varying quantities.
In the intermediate region, the fluid present is that of saturated oil, consisting of dissolved gases within it. The intermediate region of the pipe is also important for two reasons:
- Firstly, this is the region in which the first liquid portion of the fluid is present.
- Secondly, the intermediate region plays an important role in the structural considerations of the pipe. We must note, that the saturated fluid within the pipe of this region is experiencing a constant pressure at a specific temperature. Depending on the composition of the fluid, the pressures that need to be exerted may increase or decrease. Therefore, the pipes used need to be of a certain structural integrity to be able to handle these large amounts of pressure.
The last region in the constitution of crude oil is that of the bottom zone, which consists of the second liquid portion of the fluid, most oftenly known to be water. The reason for the separation of liquids into the two different regions is due to the fact that oil (first liquid region) and water being immiscible fluids. The bottom also consists of a rather large amount of inorganic salts that are dissolved into the water. The dissolved compounds are often chlorides as well as sulfates, with a combination of certain elements, such as that of sodium, potassium, calcium and even manganese.
Upon analysis of crude oils, it was noted that the compositions vary slightly in terms of the different types of components. The following table indicates the ranges that are usually confined to when producing crude oils:
| Element | Percentage by Weight (%) |
|---|---|
| Carbon | 83,9 - 86,8 |
| Hydrogen | 11,4 - 14,0 |
| Oxygen | 0,5 |
| Nitrogen | 0,11 - 1,70 |
| Sulphur | 0,06 - 3,00 |
| Metals (V,Ni,etc) | 0,03 |
In the explanation of the individual regions of crude oil, you may have come across the word hydrocarbons quite often. Hydrocarbons, as explained in one of my previous posts, is a chemical compound that has a primary chemicals skeleton/ composition that consists of carbon and hydrogen of different amounts. The amount of carbon atoms that are combined to hydrogen atoms may vary. When this occurs, different branches of hydrocarbons are identified. Hydrocarbons start from compounds as simple as methane (which consist of a single carbon atom that is chemically joined to 4 hydrogen atoms), to large complex structures such as molecules consisting of more than 60 carbon atoms. A generalized pattern was identified by scientists, which allowed for the different types of hydrocarbons to be grouped. The grouping of these branches is a function of the amount of carbon atoms present, the amount of hydrogen atoms present, as well as the nature of the bonds between these atoms.

Hydrocarbon Branches (self image)
Judging by the branches of hydrocarbons, it is important to note that all of these branches are present in crude oil. There are many more branches of hydrocarbons, but the major branches that are present predominantly in crude oils are:
n-alkanes
This branch of hydrocarbons are known to be the most basic form of all branches. There are also referred to as the building blocks of organic chemistry, as explained in one of my previous posts, due to the fact that all other branches of hydrocarbons are derivatives of this branch. Also referred to as normal or straight chain paraffins, n-alkanes are saturated hydrocarbons that form via open chains.
iso- alkanes
This branch of hydrocarbons are often known as branched chain paraffins or iso-paraffins. Similar to that of straight chain paraffins, this branch of hydrocarbons are also saturated but consist of one or more side chains of the alkyl functional group. The magnitude of branched chain paraffins increases as the fraction of the molecular weight of the crude oil increases.
Naphthenes
Naphthenes are molecules that consist of one or multiple rings that are saturated and possibly consist of alkyl side chains. These side chains predominantly are of a 5 carbon of 6 carbon atom composition, which is mainly used in the petroleum industry. Naphthenes is also known by its more common names of cycloalkanes and more renowned, cycloparaffins.
Aromatics
Also known as arenes, aromatics are hydrocarbons that consist of at least one benzene ring in its chemical composition. Benzene consists of 6 carbon atoms and are of extreme importance in the chemical industry as benzene, along with other molecules such as toluene, xylene and even ethyl, form an important part in the production of gasoline.
Mixed naphthenes aromatics
As the name implies, this branch of hydrocarbons is a compound that consist of aromatic rings, naphthene rings as well as side chains consisting of the alkyl functional groups, in the same molecule. This branch of hydrocarbons are often found in molecules that have a high boiling fraction such as kerosene fractions, and even certain oils such as gas oil and lubricating oils.

Refining of Crude Oil
This brings us to the end of part 1 of crude oils. This post has introduced to us the organic compound within crude oil as well as the different regions in which crude oil consist of. For part 2, I will be introducing and speaking about the non-hydrocarbon compounds/inorganic compounds of Crude Oil composition.
Images are linked to their sources in their description
The End
References:
[1]https://www.investopedia.com/terms/c/crude-oil.asp
[2]https://en.wikipedia.org/wiki/Petroleum
[3]https://www.foc.co.jp/en/special/03.html
[4]https://en.wikipedia.org/wiki/Chloride
[5]https://en.wikipedia.org/wiki/Sulfate
[6]https://en.wikibooks.org/wiki/Organic_Chemistry/Alkanes
[7]https://www.thoughtco.com › ... › Science › Chemistry › Chemical Laws

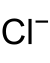
It seems a bit excessive to use hyperlinks for words such as eons, source, energy, matter etc.
True sir, but i thought that it would be able to give the reader a better understanding incase they got confused lol, but i do understand what you mean. I will take that into consideration in my newer posts