The Human body Scavengers:- Free radical vs Antioxidant system Part: -1
Hope you all are doing well!!
Today I will be discussing on an intrinsic scavenger system of the Human body.I am sure you must have known about it, but I will be just trying to boost some of your knowledge regarding it.Hope you will enjoy reading it!!
Antioxidants
Antioxidants are molecules that inhibit the oxidation of other molecules.These are man-made or natural substances that may prevent or delay some types of cell damage.They are found in many foods, including fruits and vegetables and are often available as dietary supplements.They scavenge and neutralize free radicals.
What are these free radicals?
The process of oxidation in the human body damages cell membranes and other structures, including cellular proteins, lipids, and DNA.When oxygen is metabolized, it creates unstable molecules called free radicals which steal electrons from other molecules, causing damage to DNA and other cells.So free radicals are any molecules, atoms, or ions with unpaired valence electrons(excluding some exceptional free radicals, which are with paired electrons), which are chemically reactive towards other substances, or even towards themselves i.e. their molecules often dimerize or polymerize if they come in contact with each other.
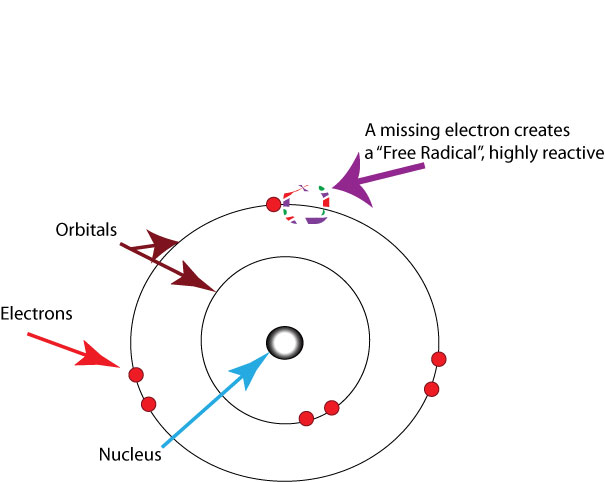
image source
Generation of free radicals:-
Free radicals may be generated within cells in several ways:-
- The reduction-oxidation reaction that occur during normal metabolic processes results in production of superoxide anion(one electron), hydrogen peroxide(two electron), hydroxyl ions(three electrons)
- Absorption of radiant energy such as UV-rays, X-rays hydrolyze water into OH and H free radicals
- Rapid bursts of Reactive Oxygen Species(ROS) are produced in activated leukocytes during inflammation
- Enzymatic metabolism of exogenous chemical or drugs results in the generation of carbon trichloride free radicals from break down of carbon tetrachlorides.
- Transition metals such as Iron and Copper can donate or accept free electron during intracellular reactions and catalyze free radical formation.
- Nitric oxide(NO), generated by endothelial cells of blood vessels, macrophages, neurons, and other cell types act as free radical and often convert to highly reactive peroxynitrite anion
How does body combat these free radicals?
Cells have developed multiple enzymatic and non-enzymatic mechanisms to remove free radicals and thereby minimize injury.These include the following:-
- Antioxidants either block free radical formation or inactivate free radicals.Examples are the lipid-soluble vitamins E and A as well as ascorbic acid(vitamin C) and glutathione in the cytosol.(I will be discussing about the Antioxidant system in detail in next part)
- Iron and Copper can catalyze the formation of ROS.Under normal circumstances, the reactivity of these metals is minimized by their binding to storage and transport proteins(eg., transferrin, ferritin, lactoferrin, and ceruloplasmin), which prevents these metals from participating in reactions that generate ROS.
- A series of enzymes acts as free radical-scavenging systems and breaks down hydrogen peroxide and superoxide anions.These enzymes are present near the site of generation of oxidants and includes the following:-
1.Catalase, present in peroxisomes, decomposes Hydrogen peroxide into water and molecular oxygen.
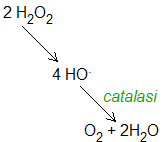
image source
2.Superoxidase dismitases(SODs) are found in many cells type both in mitochondria and cytosol and convert superoxide anions into hydrogen peroxide.
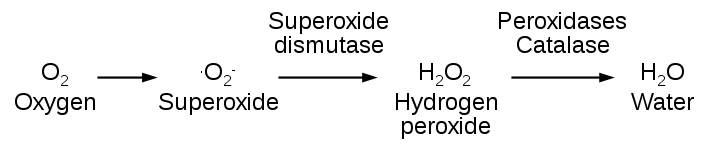
image source
3.Glutathione peroxidase protects against injury by catalyzing free radical breakdown.
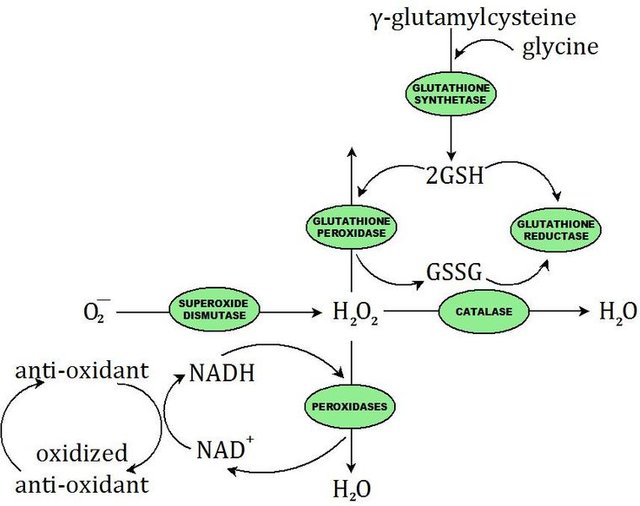
image source
{Above figure show:-Structure of glutathione(GSH), Actions of glutathione peroxidase enzyme. G-SH(reduced glutathione); G-S-S-G(oxidized glutathione), Glutathione-mediated reduction of hydrogen peroxide by NADH}.
Pathological consequences of free radical!
The production of ROS is increased by many injurious stimuli.These free radicals are removed by spontaneous decay and by specialized enzymatic systems.Excessive production or inadequate removal leads to accumulation of free radicals in cells, which may damage lipids, proteins, and deoxyribonucleic acid (DNA), resulting in cell injury.
The effects of ROS and other free radicals are wide-ranging, but these three reactions are particularly relevant to cell injury:-
1.Lipid peroxidation in membranes which cause an autocatalytic chain reaction resulting in extensive membrane damage.
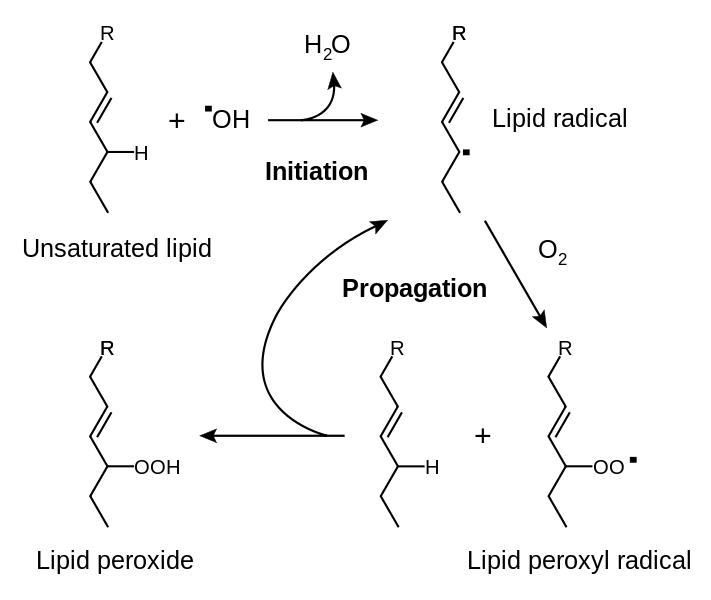
image source
(In the figure above we can see that in presence of oxygen, free radicals may cause peroxidation of lipids.Oxidative damage is initiated when fatty acid of the membrane is attacked by oxygen-derived free radicals, particularly by hydroxyl ion)
2.Oxidative modification of proteins
Free radicals promote oxidation of amino acid side chains, formation of covalent protein-protein cross -lins, and oxidation of the protein backbone.This results in a conformational change in the active sites of enzymes and the proteins itself and enhance proteosomal degradation of the misfolded proteins.
3.Lesions in DNA
Free radicals are capable of causing single and double-strand break in DNA, cross-linking of DNA strands, and formation of adducts.
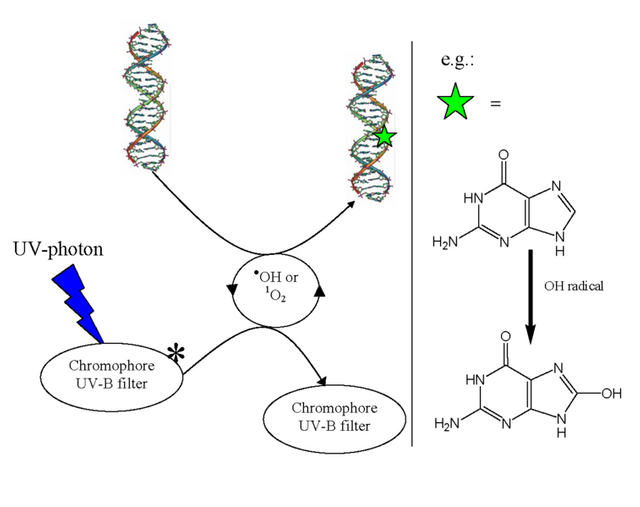
image source
(In the figure we can see the indirect damage to DNA resulting from UV-rays generating free radicals)
The traditional thinking about free radical was that they cause cell injury and death by necrosis.But the new concept has illustrated that free radicals can trigger apoptosis(cell suicide) as well.But free radicals aren't only the deadly molecules as we think.In fact, it can sometimes serve in an important physiological functions when produced under physiological conditions in the 'right' dose.
This is all about free radical in this part.The more about Antioxidants will be discussed in the next part of my blog.Hope you enjoyed reading it.
References:-
1.Robbins and Cotrans Pathological Basis of Disease(South Asia Edition)
2.Harper’s Illustrated Biochemistry 29th Edition
3.Lippincott’s Illustrated Review of Biochemistry 5th Edition
4.https://en.m.wikipedia.org/wiki/Radical_(chemistry)
5.https://www.sciencedaily.com/releases/2011/02/110228090404.htm
#Steemstem
The Steemstem team has been working for over one a year and now to promote informative S-Science T-Technology E-Engineering M-Mathematics post on steemit.The project aim is to build a community of science and technology lovers on steemit and aids in fostering the growth of posts and blogs.To know more about the project you can join the channel in discord here.
Have a nice day!!
Great content... Very educative and enlightening
Thanks for reading @emmanuelzod.
Excellent post even if my chemistry knowledge is a bit limited.
High quality article. Well done.
Thanks for the appreciation.@irelandscape
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Amit from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.