Maxwell's Zombie: Duncan's Perpetual Machine
My last post ended with the death of Maxwell's demon. The second law of thermodynamics was saved when Benette put the final nail in the coffin in 1937. At the end of 20th century, D. P. Sheehan published a paper, Dynamically maintained steady-state pressure gradients [1]. The paper wasn't intended as an attack on the second law of thermodynamics but a different picture was revealed when Duncan made a comment on Sheehan's paper, Comment on ‘‘Dynamically maintained steady-state pressure gradients’’ [2]. Duncan pointed out that the findings made by Sheehan contradicted with the second law of thermodynamics. Yes, the demon is back from the dead! This gave rise to Duncan's Paradox which I will be talking about in this post.

Dynamically maintained steady-state pressure gradients
A pressure gradient is basically a difference in pressure. Change in hydrostatic pressure with the depth of a liquid is an example of a pressure gradient. Another example is the atmospheric pressure gradient. You may already know that the atmospheric pressure decreases as the altitude increases. This pressure gradient is a static steady-state pressure gradient. Static because it is in equilibrium with gravity and steady-state because it doesn't change with time. Dynamically maintained steady-state pressure gradient(DSPG) is another type of pressure gradient formulated by D. P. Sheehan in 1998. DSPG occurs due to difference in disassociation and desorption rate of chemical species A2 and A on two surfaces S1 and S2. Let's break it down!
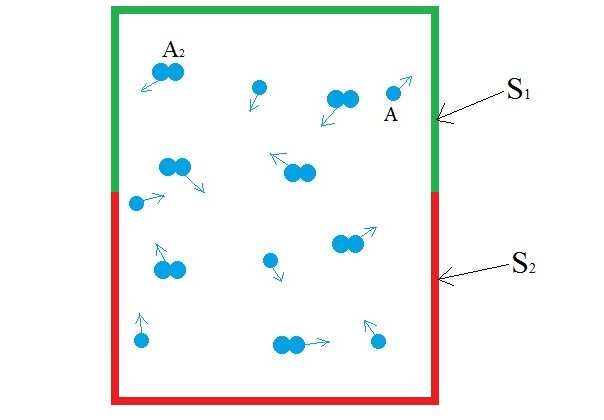
Consider a blackbody cavity whose walls are made up of two distinct surfaces, S1 and S2. The cavity is filled with a diatomic gas A2 (for eg. Hydrogen(H2)). The gas is in low pressure so that the gas-surface collision is more prominent than the gas-gas collision. The two surfaces are chemically distinct in such a way that S1 dissociates and desorbs A2 more effectively than A and the surface S2 dissociates and desorbs A more effectively than A2. Here's what happens at the molecular level.
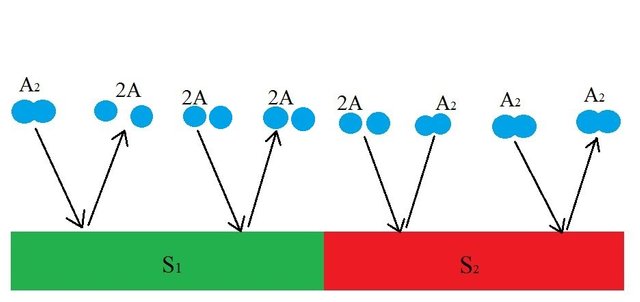
As you can see, the diatomic molecule is broken down and desorbed into two molecules of A (2A) by S1 but if two molecules of A comes in contact with S1, nothing happens. Meanwhile, the S2 surface is chosen such that it combines and desorbs two molecules of A (2A) into a diatomic molecule(A2) The dynamic process of disassociation and association of molecules maintains a chemical equilibrium:
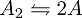
A is a monoatomic gas, its thermal velocity is 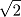 times the velocity of A2. According to the conservation of linear momentum, a net force acting on the surface S1 is greater than that in S2. Hence, a pressure gradient is created between the two surfaces. This is the basic principle behind Dynamically maintained steady-state pressure gradient. One thing to note is that a thermal gradient is also created. The reaction on S1 is an endothermic and the reaction on S2 is exothermic, this results in a temperature gradient between two surfaces. Whether its a pressure gradient or a thermal gradient, the second law of thermodynamics doesn't allow this to happen.
times the velocity of A2. According to the conservation of linear momentum, a net force acting on the surface S1 is greater than that in S2. Hence, a pressure gradient is created between the two surfaces. This is the basic principle behind Dynamically maintained steady-state pressure gradient. One thing to note is that a thermal gradient is also created. The reaction on S1 is an endothermic and the reaction on S2 is exothermic, this results in a temperature gradient between two surfaces. Whether its a pressure gradient or a thermal gradient, the second law of thermodynamics doesn't allow this to happen.
Duncan's Perpetual Machine
Duncan's comment on DSPG was that the pressure or thermal gradient can be utilised to do mechanical work. His idea is to apply the material S1 and S2 on the opposite side of a turbine blade. Due to the pressure difference on the surfaces, the turbine rotates which can be used to perform work.
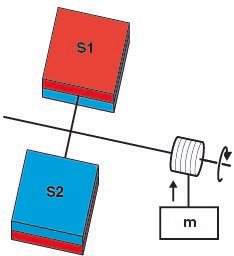
The apparatus has to be enclosed by a heat bath (not shown in the figure). The molecules (A2 and A) receive thermal energy from the heat bath and maintains a pressure gradient between the two surfaces.T he pressure difference rotates the turbine which can be used to run to run a generator to produce electricity. Basically, the molecules use thermal energy from a source and convert it into mechanical work but the second law of thermodynamics doesn't allow this to happen.
The Kelvin–Planck statement (one of the three ways to state the second law of thermodynamics) states that it is impossible for a thermodynamic cycle to operate that extracts heat and use all of it to do work. It means that a 100% efficient heat engine cannot exist but Duncan's apparatus converts heat energy from the thermal reservoir into an equivalent amount of work. The same thing can be observed in the case of temperature gradient.
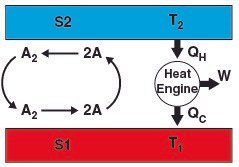
The reaction at S1 (A2 -> 2A) is endothermic and the reaction at S2 (2A -> A2) is exothermic. This creates a temperature gradient between the two surfaces. This contradicts the second law of thermodynamics. Either the second law has to be wrong or there must be some fallacy in the formulation of DSPG. Questioning the validity of the second law is the greatest sin a physicist could commit. I mean, come one, its the most fundamental law of nature. Why would anyone bother to check its validity? Actually, Sheehan conducted an experiment and the results are somehow controversial.
Expermental Verification
Sheehan, who formulated DSPG back in 1997, conducted an experiment in 2014 to check its validity [3]. The experiment was done to measure the temperature gradient rather than the pressure gradient because it's difficult to measure small pressure gradients.
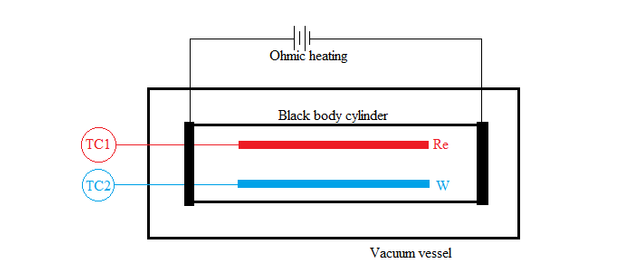
Rhenium(Re) and Tungsten(W) coated thermocouples were used as surfaces S1 and S2 respectively. They were then connected to two thermocouple gauses TC1 and TC2 to measure the temperature. To create blackbody condition, the core cylinder, on which the metals were placed, was wrapped with foils of either tungsten or rhenium. The core cylinder was continuously heated to maintain an isothermal condition inside it and then placed inside a vacuum sealed vessel to prevent heat losses. Hydrogen gas (H2) was used as the working gas because of its ability to react with high-temperature refractory metals. Instead of just measuring the temperature difference for hydrogen gas, the experiment was conducted for Helium too. According to the second law of thermodynamics, the temperature difference both gases have to be zero-i.e.,

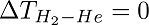
The experiment was conducted over a wide range of temperature(1,000 ≤ T ≤ 1,950 K) and pressure(0.1 ≤ P ≤ 10 Torr) and yes, the temperature difference was observed as predicted by Sheenan and Duncan!! The largest measured temperature difference was 126K (-147.15 °C). This may look small but this temperature difference is equivalent to a Carnot engine with the efficiency of 60%. The results contradict the second law of thermodynamics. They (Sheenan et al.) concluded the paper as:
In summary, Duncan’s temperature difference has been experimentally measured via differential hydrogen dissociation on tungsten and rhenium surfaces under high-temperature blackbody cavity conditions. We know of no credible way to reconcile these results with standard interpretations of the second law. [1]
This means the second law is dead?
Final words
Just like any other physical law, the second law of thermodynamics doesn't have any general theoretical proof. It is completely based on what we observe in nature. Whether it's a cup of cooling coffee or the expansion of the universe, the second law holds. But what about the results of the experiment? The results of the experiment are indeed mind-boggling but we cannot say that it is a conclusive proof of violation of the second law until the experiment is repeated with the same result. Personally, i don't think there is any possible argument against the second law. It is so fundamental that the moment someone disproves it, the universe will cease to exist.
The second law of thermodynamics has the same degree of truth as the statement that if you throw a thimbleful of water into the sea, you cannot get the same thimbleful of water out again. [4]- James Clerk Maxwell
References:
[1] Sheehan, D. P. (1998). Dynamically maintained steady-state pressure gradients. Physical Review E, 57(6), 6660.
[2] Duncan, T. L. (2000). Comment on “Dynamically maintained steady-state pressure gradients”. Physical Review E, 61(4), 4661.
[3] Sheehan, D. P., Mallin, D. J., Garamella, J. T., & Sheehan, W. F. (2014). Experimental test of a thermodynamic paradox. Foundations of Physics, 44(3), 235-247.
[4] Sheehan, D. P. (2007). The second law of thermodynamics: Foundations and status. Foundations of Physics, 37(12), 1653-1658.
1. Cápek, V., & Sheehan, D. P. (2005). Challenges to the second law of thermodynamics. Dordrecht: Springer.

SteemSTEM is a community project with the goal to promote and support Science, Technology, Engineering and Mathematics on the Steem blockchain. If you wish to support the steemSTEM project you can:
Contribute STEM content using the #steemstem tag | Support steemstem authors | Join our curation trail | Visit our Discord community | Delegate SP to steemstem
Great Maxwell! I always enjoy reading about this. Was it with you I discussed that topic months ago?
Note that in the picture :"A zoomed in picture of the DSPG Apparatus", I would replace the single blob representing 2A molecules by two distinct blobs, on the S1 side. This will make it easier to understand.
Personally, I would like to wait and see for an experimental confirmation. And even if this is true, there must be another explanation. There are few principles that I am not ready to send to the bin, like energy conservation. the second law of thermodynamics. etc.
@lemouth Yes sir, we discussed this a couple of months ago. Indeed, there has to be some explanation for the pressure/temperature difference. We just can't disregard a fundamental law such as the second law.
(i corrected the image :D)
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
Splendid use of oxymoron in inner conflict of Doccon.
Hi @anevolvedmonkey!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Congratulations @anevolvedmonkey! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Congratulations @anevolvedmonkey! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Congratulations @anevolvedmonkey! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!