Gene Drive and the Engineering of Extinction – Part I

What do you think is the most dangerous animal on Earth? Forget about predators with shiny fangs, the animal responsible for the highest number of human deaths is so common you probably already crossed its path multiple times:

Mosquitos are responsible for several million human deaths each year and hundreds of millions of new cases of the terrifying set of diseases they carry: Malaria, West Nile virus, Zika… Vaccines are not yet available for some of these diseases and for others they are not fully efficient or readily available in areas at risk. Malaria is probably the best example: even though the mortality due to the parasite is decreasing thanks to multiple vaccination campaigns, the limited efficiency of the vaccine and the apparition of resistance against Artemisinin, one of the main molecule used to cure the disease, have recently raised concerns. The World Health Organization even considers that 50% of the world population is currently at risk for the disease, and with global warming the problem could soon expand further North.
In consequence, an important part of the effort to control the spread of these epidemics relies on the control of mosquito population. A famous example is the widespread use of Dichlorodiphenyltrichloroethane or DDT in the 40s and 50s. This insecticide was sprayed by plane over humid areas to kill mosquito eggs and larvae and allowed to largely reduce Malaria mortality in certain regions. However, the important toxicity of the molecule, its disastrous effect on wildlife (particularly on birds feeding from contaminated insects), as well as proven carcinogenic effect and the apparition of resistance leaded to a global ban of DDT in the 70s.

Another strategy, first developed to control fruit flies is the sterilization of male insects. To do so, mosquitos are bred in a lab, males are then separated from females and sterilized (often by irradiation) before being released in the wild. These males will then compete with wild males to mate with females, which will result in a non-viable offspring for these females and potentially reduce the global population.
While this approach has the advantage of not releasing harmful chemicals in the environment and to target a single species without affecting other insects, it has important limitations. To observe an effect on a whole population, it requires the constant release of new sterilized individuals, which are often less competitive than wild ones for reproduction and have a shorter lifespan because of the treatment they underwent. To overcome these limitations, a new method is now being experimented which uses a more sophisticated and direct modification of the mosquito genome, but first let’s talk about genetic selfishness.
The selfish gene
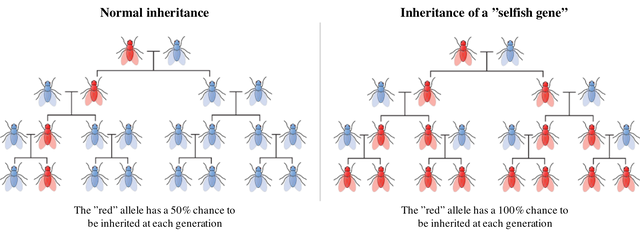
If you read my previous article on gene editing with CRISPR/Cas9 (part I, part II), you know that in Eukaryotes a DNA double strand break could be repaired by the cell through different mechanisms. One of these repair pathways, called Homology Directed Repair, uses the intact chromosome of the pair as a template to precisely repair the damaged region. In eukaryotic cells, chromosomes are indeed present in pairs, which means that every gene is present in two copies called alleles that could be identical or slightly different. Each allele is inherited from one of the parents and each one has a 50% chance of being transmitted to the offspring of the considered individual.
However, some genes found in nature can hijack the repair machinery to stack the odds in their favor, they are thus called “selfish” genes. Let’s consider an individual who inherited one copy of a selfish gene from one of his parents. The selfish allele is thus present on only one chromosome of the considered pair and the organism is called heterozygous for this gene. The selfish allele encodes an enzyme called homing endonuclease whose function is to recognize and cut very specific DNA sequences in the genome (just like the CRISPR/Cas9 system but with only one specific target region).
In our case, the homing endonuclease specifically recognizes and cuts the allele on the chromosome that does not contain the selfish gene. Since the cell needs to use the intact chromosome of the pair as a template to repair the broken region and this same chromosome contains the selfish gene, this results in the copying of this gene to the broken chromosome.
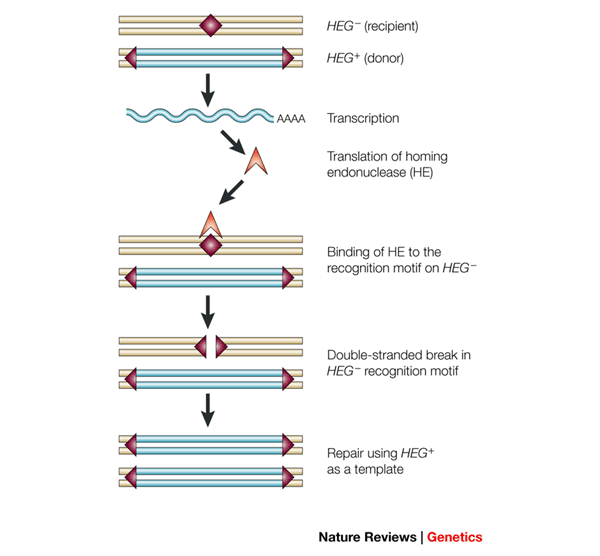
“Selfish” genes are thus able to induce their own propagation from one chromosome of the pair to both by damaging the chromosome that does not contain the gene and presenting themselves as the template to repair the break. The interesting thing with this mechanism is that selfish genes can thus change their probability of transmission to the offspring from 50% to 100% by artificially changing from heterozygote to homozygote at each generation until they are present in the whole population.
In the second part of this article, we will see how this mechanism could be used as a deadly weapon to potentially destroy a whole species.
Sources and further read:
- Homing endonucleases
- A resource page of the WHO on Malaria
- To read more about the historical use of DDT
- A review on the evolutionary role of selfish elements
- To read more about the sterile insect technique
Being A SteemStem Member
Interesting. During the process of damaging the other allel, are there any negative side effects for the 'selfish gene'? Does it sometimes lead to self damage or termination of the whole cell? Or does it always execute and give it a 100% chance transmission when it is attempted?
Hi @kilbride, that's a very good question. I did not find any study on the question, but my guess is that these elements are generally integrated in non-coding regions of the genome or in gene introns, which reduces the risks of potential gene disruption during the transfer. I think that any homing endonuclease that would target an essential gene would have been eliminated during evolution.
The transmission efficiency is definitely not 100%, since homologous recombination is active only during certain phases of the cell cycle and not in every cell type, I think there is a significant proportion of cutting events that simply lead to the mutation of the recognition site of the enzyme, preventing recutting and the "copying" of the gene. The last figure is a bit misleading because too simplified, thank you for raising this question :)
Hi @kilbride and @carlgbush. I perform CRISPR/Cas9 gene editing in human pluripotent stem cells, so I am familiar with these topics. Yes, self damage and apoptosis (termination of the whole cell, "programmed cell death") can occur. Pretty much nothing in biology is 100%, so the desired editing events, particularly transmission of the gene of interest, is a fairly rare event. But, there are many things to consider when designing your gene targeting strategy. My understanding is that gene drive in its current form is more used to remove a gene from a population. Removing a gene is a much easier process than making a specific change because you are only relying on the repair machinery of the cell to accidentally repair the double stranded DNA break with an insertion or deletion (termed indel) of one or a few additional/less DNA bases, thus causing a frameshift mutation and essentially removing a gene product.
I would think to actually integrate the "homing endonuclease" (really a Cas9 and a few guide RNAs targeting the region of the genome that you want) directly into the gene that you want to remove would be most efficient, particularly if you were to add a stop codon just downstream of your homing endonuclease. So if the repair process is through homologous recombination, you will end up removing the gene that you wanted to target. However, if the repair process is non homologous end joining (NHEJ), you'll still remove the targeted gene of interest because you'll hopefully have created an indel, rendering the gene product functionally useless.
Hi @fighton, thank you for taking time to comment, you are completely right about gene drive. In this article I only talk about the natural occurrences and mechanisms of homing endonuclease, that's what @kilbride question was about. I will talk about Cas9 and how this mechanism is used for gene drive in the next article :)
Great post :)
I've studied genetics and genomics a lot but never heard of this concept.
This is a really interesting concept, I wonder if this is related to the roughly 98% of our genome that's non-coding :/
Also seems like a good way for endegenous retrovirsuses to lie dormant, multiply and the destroy us all :'(
Congratulations @carlgbush! You received a personal award!
Click here to view your Board
Do not miss the last post from @steemitboard:
Congratulations @carlgbush! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!