Determination of the Physical Variables Involved in the Electrodeposition Phenomenon of Nickel and Chromium Metals.
Electroplating
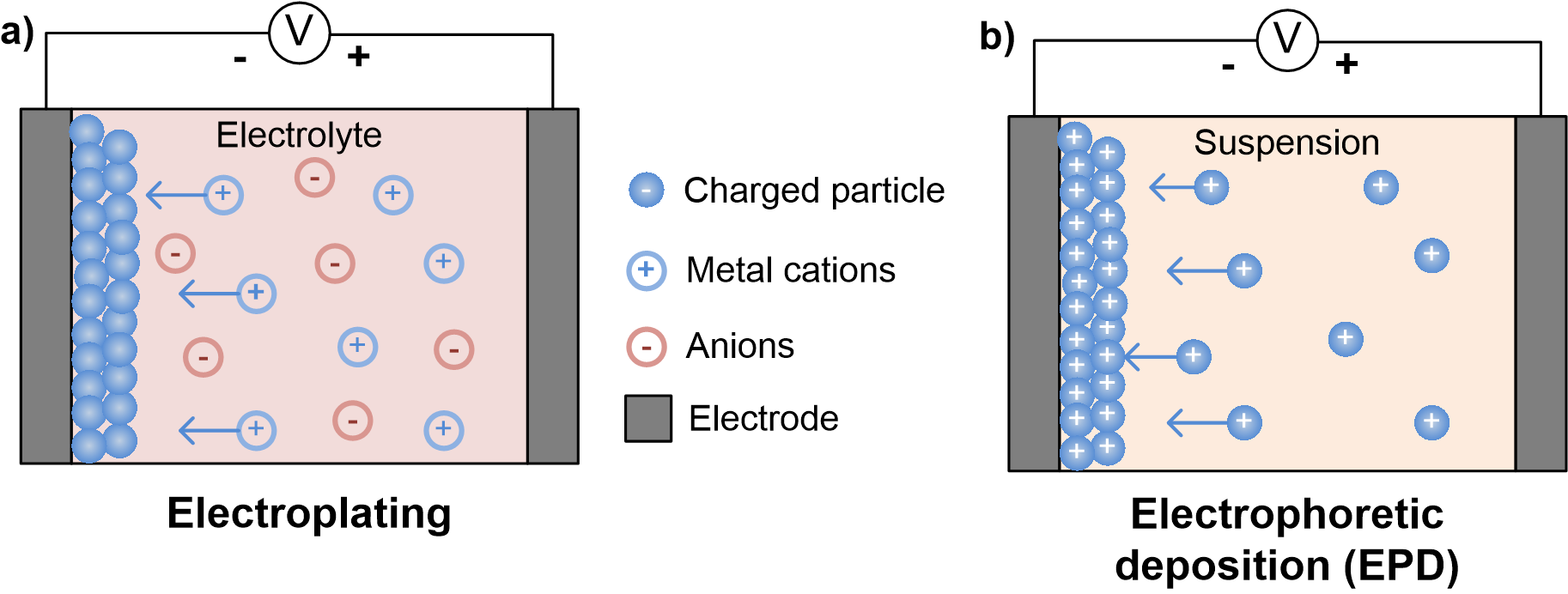
From the point of view of physics, it is the electrodeposition of a metal on a surface to improve its characteristics. This is achieved by providing hardness, duration, or both. Another of the important applications of electroplating is to reproduce by electrochemical means objects of very fine details and in very different metals. The electrochemical process can be summarized as the transfer in the form of metal ions from an anode to a cathode through a liquid medium (electrolyte), composed mainly of salts, as a result of applying a direct current in a device or reactor that constitutes a circuit electrically.
The deposition of metal ions on the surface prepared to receive them is carried out faithfully following the details that make up this surface, the molecules coalescing as they lose their positive charge and strongly adhering to each other, thus forming a metallic surface, with characteristics corresponding to the metal that makes up.
Source
Electric chrome.
The bright or decorative chrome are thin layers of chrome that are deposited on copper or nickel to improve the appearance of some objects. The famous nickel plating of bumpers and other car trims usually consists of a layer of nickel finished with a chrome flash of a few microns in thickness. The color of the chromium is more bluish and reflective than nickel and is much more resistant to corrosion because it immediately forms a thin and imperceptible layer of oxide that protects the metal.
Chromium has little covering power, even less if the layers that are deposited are as thin as one micron. Therefore, the surface to be covered must be well polished, shiny and decreased since the chrome will not cover any imperfection. It is for this reason that often the pieces that are chromed with a decorative object are covered with copper and nickel before they are chromed. Chromium is applied well to copper, nickel, and steel, but not to zinc or smelting.
Nickel-plated
Nickel is a metal very similar to iron, in fact chemically studied together and form a group. Along with the cobalt, the three are "ferromagnetic". It is malleable ductile and it resists corrosion quite well worse than stainless steel and worse than chrome. It is a color similar to iron but a little more yellowish and less gray. When chrome is applied to decorative objects, it is usually done on a thicker nickel layer.
Faraday's laws of electrolysis
The laws that describe electrolysis are:
The chemical change produced in electrolysis is proportional to the charge of electricity that passes through the cell.
The load required to deposit or release a mass m is given by Faraday's law.
Faraday's law of electrolysis in the modern form:
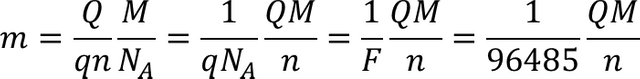 )
)Where:
m is the mass of the substance produced in the electrode (in grams),
Q is the total electric charge that passed through the solution (in coulomb),
q is the electron charge = 1.602 x 10-19 coulomb per electron,
n is the valence number of the substance as ion in the solution (electrons per ion),
F = qNA = 96485 C.mol-1 is the Faraday constant,
M is the molar mass of the substance (in grams per mole), and
NA, is Avogadro's number = 6.023 x 1023 ions per mole
Electrolysis of salts
Certain substances, (acids, hydroxides, salts and some dissolved or melted metal oxides) are conductors of electricity at the same time that they decompose when the electric current passes, these so-called electrolytes. This phenomenon is called electrolysis and is basically a process of oxidation-reduction that develops "not spontaneously" that is, a set of transformations that imply an increase in free energy of the system, and therefore, requires the completion of the competition for an external force of energy. Like electrochemical cells, an electrolysis reaction can be considered as the set of two half reactions, one anodic oxidation and one cathodic reduction.
When we connect the electrodes with a power source (direct current generator), the electrode that is attached to the positive terminal of the generator is the anode of the electrolysis and the electrode that is attached to the negative terminal of the generator is the cathode of the electrolysis.
The reactions that take place in the electrodes of the electrolysis are generally determined by energy laws, as well as in the cell, the reaction in each electrode is the one that corresponds to a reaction that produces the maximum decrease of free energy, in the electrolysis it they will produce the reactions that correspond to a total reaction that produces the minimum increase of free energy.
In the case of electrolysis of aqueous solutions of various electrolytes, the reactions that take place in the anode must be chosen according to the principles of possible oxidation at the anode, more than a possible reduction in the cathode, because in addition to the species ionics produced by electrolytes are present water molecules and it can be oxidized and reduced in a similar way to salts.
For example, during the electrolysis of the CuSO4 solution (with smooth platinum electrodes) on the cathode, the separation of the metallic copper is observed. On the other hand, on the anode, the water molecules leave their charge and not the SO4 ions. The electrolysis scheme is as follows:
On the cathode (-): 2Cu2+ + 4e → 2Cu ↓
On the anode (+): 3H2O → 2H3O+ + 1/(2O2) ↑ + 2e
On the anode, in this case, oxygen is removed, which is removed as a gas, and in the solution, in the vicinity of the anode, H-ions accumulate, which may be present only in an equivalent amount of some anions. Such anions are SO_4 which move during electrolysis to the anode and accumulate in the vicinity of this together with the H ions. Therefore, next to the anode, sulfuric acid is also formed in addition to oxygen (as corresponding ions). say, the solution is acidified.