Harnessing the potential benefits of recombinant DNA technology
It is very possible to genetically modified a bacteria cell, giving it an ability to detect some abnormality in the body. Surprised?? Another more fascinating aspect of this discourse is the fact that, the modified bacteria cell is attached to an ingestible chip and then configured to produce some signals that are readable invitro (outside the body) and these produced signals help diagnose a disease condition in the body
 [Ingestible bacteria on a chip device, image credit :Lillie Paquette/MIT, MIT]
[Ingestible bacteria on a chip device, image credit :Lillie Paquette/MIT, MIT]The big questions
The big question now is how? How feasible is this? Is there no possibility that these bacteria cell might later turn out to be virulent (harmful)? What is the mechanism of action? How long will these bacteria cell remain in the body without reverting? The so called device it is placed in, how durable is it? How does this device function? What keeps the device active in the stomach without external power? All this questions will be addressed as we move on.
Our discussion won't be balanced if we neglect the concept of genetic engineering- the hall mark and basis upon which this new discovery lies on.
Genetic engineering
The advent of genetic enginering in the field of science has really paved more pathways for more and more improvement in ways of harnessing desirable characteristics and attribute from a potentially harmful living organism. Generally, scientist are never scared of exploring a creature be it harmful ones or not, they dare to find out what makes such creature the way it is.
Genetic engineering can also be referred to as genetic modification or genetic manipulation, and it's a process of intentionally altering the genetic components of an organism in a bid to produce an organism with desirable qualities. This is usually done using biotechnology.
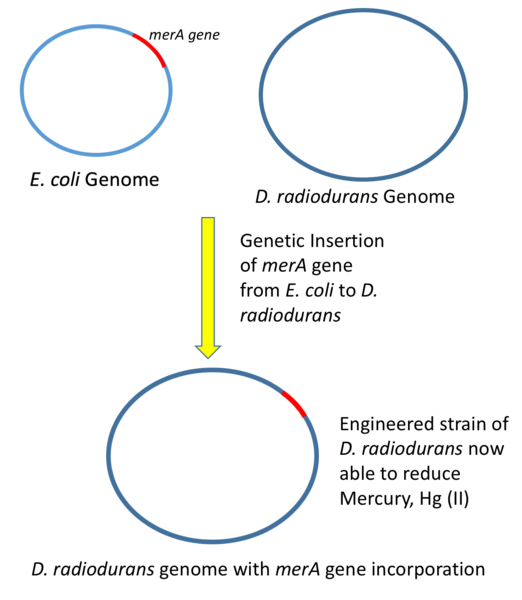
In genetic engineering, the major aim of the scientist is to systematically alter the DNA sequence (genetic make up) of a living organism and in a bid to harness and take advantage of the potential benefits that comes with the process. Take for example, the world toughest bacterium - Deinococcus radiodurans, a bacterium with the ability of withstanding and resisting ionising radiation and also with the ability of repairing DNA damage.
Due to its high potential, Deinococcus radiodurans was genetically modified to digest solvents and also reduce heavy metals like mercury in radioactive areas. The gene of interest (mercuric reductase gene -merA) was isolated from E. coli and inserted into D. radiodurans, thus giving it the ability to also reduce heavy metals in addition to its other potentials. Today, they are used for decontaminating areas with radioactive waste.
The process of genetic engineering (GE), requires great deal of carefulness as the process could end up yielding a product with negative or positive features. Either way, let's critically look at how this is done.
How is GE carried out?
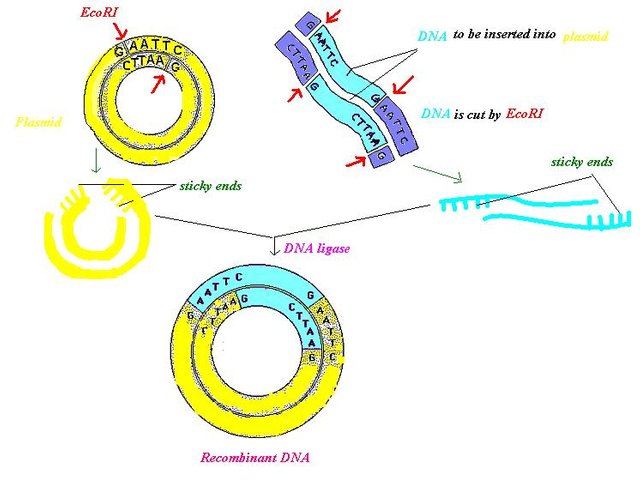
The first step in GE is the isolation of the gene of interest from a foreign DNA using an enzyme known as restriction endonuclease and amplification of these gene of interest in a vector - plasmid (a common vector for holding genes). The amplification (creation of multiple copies of the same gene) is done using a technology known as PCR (polymerase chain reaction). For the benefit of doubt, vectors are gene carriers with origin, clonning and insertion sites. They help hold gene in place for replication. They are piece of DNA that are capable of growing independently. The most commonly used vectors are the bacterial plasmids and viral phage.
The amplified gene in the plasmid is also cut out using restriction endonucleases. A well known restriction endonuclease is the EcoRI. These restriction endonucleases play the primary role of cutting gene sequences at a particular restriction site, thus producing sticky ends that will later be joined to complementary strands by an enzyme known as the DNA ligase to produce a recombinant DNA (r-DNA) .
To make it simple and short, a recombinant DNA also referred to as chimera is a product of two genes, one from that of a bacteria cell and the other from an animal, and it is done so as to produce products with better and improved features. Now that the r-DNA has been formed, the next step is to insert the vector containing the foreign DNA (r-DNA) into the host cell so that it will produce the desired products.
The Genetic transformation process
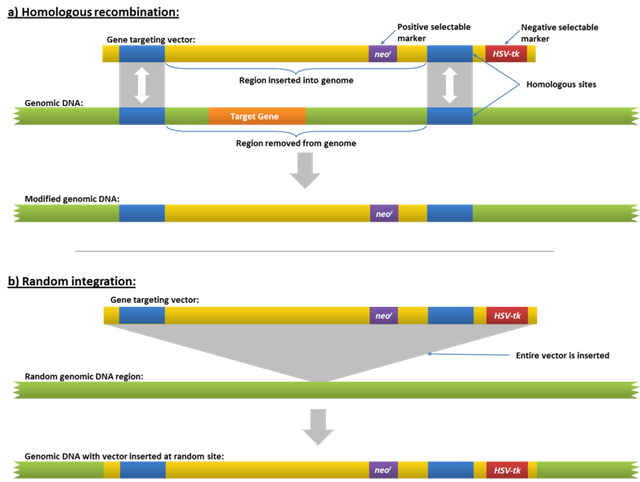
The DNA is inserted into the host organism is referred to as transformation and it is done in two ways, first is either through the random process where, it is inserted anywhere in the genome or through a specific target point in the genome of the host organism. In the process of targeting the specific region, a method known as the homologous recombination (a method of horizontal exchange of gene or genetic transfer between two different species and strains of organisms, especially virus and bacteria) is used to effect the host genome.
What the process does
The process simply knocks out the undesirable gene in the genome and then the desired gene inserted. Commonly, E. coli is the widely used host for genetic transformation, as it has been found to easily accept foreign DNA. The host cells is then cultured in a media which contains antibiotic for growth. The essence of the antibiotics is to ensure that only the bacteria with recombinant DNA grows. The products at them harvested and used for their specific purposes.
Products of GE
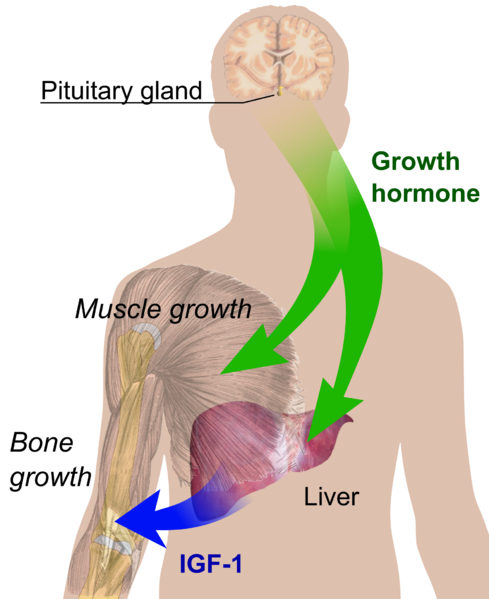
Due to the emergence of genetic engineering, biosynthetic therapeutic proteins such as insulin (used for regulating blood sugar), follicle stimulating hormones - FSH (used in fertility in humans), erthropoeitin (used for enhancing the production of red blood cells in humans), plasminogen (used in blood clotting issues) and even growth hormones have been produced in a bid to replace deficiencies and to also strengthen the immune system to fight diseases and infection.
Initially before recombinant human growth hormone (r-HGH) was made available, it was reported that this growth hormone was obtained from the pituitary glands (the master gland that controls all other secretion of hormones into the blood system) of cadavers(dead bodies). As a result of this bad practice, it resulted to patients developing a disease condition known as Creutzfeldt–Jakob disease (a degenerative brain disorder which leads to dementia and death)
Patients that suffer some diseases as a result of defiencies can now take the biosynthetic products either through injection or orally so as to reverse the negative effects suffered a result of the deficiencies. The products of genetic engineering is not limited to the above stated ones, as there are many products that have been produced as a result of GE.
GE has made it possible to produce cropss that are highly resistant to pest. Just recently, CNN released a report that a genetically modified diamondback moth has been released into the wild so as to kill and wipe out pets.
This summarises everything about genetic engineering, lets now talk about this novel - ingestible bacteria chip, product made from a combination of both genetic engineering and biomedical engineering. For more understanding about biomedical engineering, read this article BME in medicine - the concept and future prospects.
Ingestible bacteria on a chip device
Most times in medicine, the goal and aim of modern advancement is always geared to make procedures less invasive (you don't need to cut open the patient to diagnose a disease condition). The ingestible device on a chip is a brilliant innovation by scientist from massachusetts institute of technology (MIT). They were able to genetically engineer a strain of E. coli, making it capable to luminescing when they come in contact or interact with a biomarker.
How does it work?
The bacterial chip contains pro-biotic cells (living or live microorganism e.g bacteria cells indicated to be safe and provides some health benefits when consumed) that have been genetically engineered or modified to sense biomarkers that are associated with some health disease condition. These bacteria cells are incorporated into micro -wells in the device which are then trapped in a semi permeable membrane. One beautiful thing about the design of this device is that, the semi-permeable membrane only allows the diffusion of biomolecules to be sensed into the well but prevents the probiotic cells from diffusing out.
The picture below shows the device with four micro-wells where the probiotic cells are placed and behind it is the semi-permeable membrane.
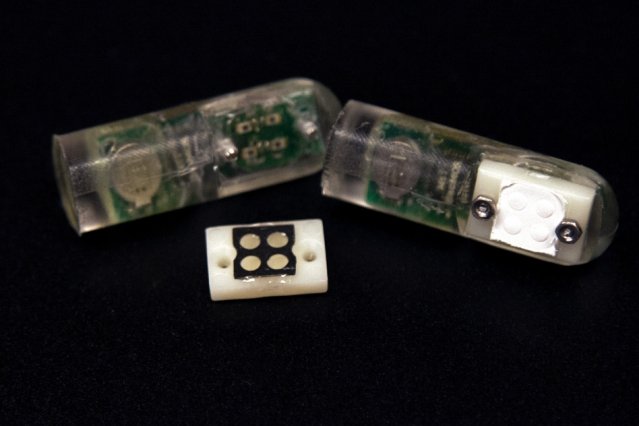 [Ingestible bacteria on a chip device, image credit :Melanie Gonick/MIT, MIT]
[Ingestible bacteria on a chip device, image credit :Melanie Gonick/MIT, MIT]Once the biomolecule (biomarker) to be sensed diffuses across and interacts with the probiotic cells, the cells become activated and they begin luminescing. The luminescence are detected by phototransistor that measures the amount of light emitted by the bacteria cells. These phototransistors then send the signals to a microprocessor which in turn sends the signals to a wireless transmitter that finally transmits the information outside the body to user cellphone. As long as the specific bio-sensor is slotted into the wells, the device has the ability of sensing as many biomarkers as possible.
Another rooms for adjustments in this device is that, there is possibility to creating many micro-wells which contains specific pro-biotics cells that are specific for different biomarkers. The possibility here is that, a single ingestible bacteria on a chip device can detect many disease conditions at once.
Normaly patients with GIT disorders are sedated and endoscopy (passing a tube through the guts to detect what the problem is, but with this devise, all the patient needs do is to just swallow the pill. This will saves cost, save the patient from spending much on diagnostic test and as well boycott the rigorous procedures of detecting what the patient is suffering.
With this ingestible device, it is easier to detect gastrointestinal problems like bleeding, ulcer and other GIT issues. Heme is one of the major component of blood released during gastrointestinal bleeding and it is used as a biomarker. Ingestible chips to be designed will detect this heme. This is how other problems are also detected.
One challenge anyone would have probably talked about is how to power the sensor and the device as a whole, but obviously this has been taken care of.
The sensor, which is a cylinder about 1.5 inches long, requires about 13 microwatts of power. The researchers equipped the sensor with a 2.7-volt battery, which they estimate could power the device for about 1.5 months of continuous use. They say it could also be powered by a voltaic cell sustained by acidic fluids in the stomach, using technology that Nadeau and Chandrakasan have previously developed
It's quite understandable that the prototype is too big to be ingested for now, but in due time, the size will be reduced drastically to micro chip that will be fully applied to clinical use. The use of this device will be a great advancement and improvement in the medicine.
Despite the advantages, there are still some gray areas that need clarification. one of which is, the possibility that the materials used in making the device won't react with the acid of the stomach, hence causing more problem for the patient. Since the prototype has been tested on pigs and it showed wether it had GIT bleeding, what's the possibility of the engineered cells surviving for long in the human guts.
That not withstanding, this is a ground breaking
innovation and will go a long way in solving the problem associated with endoscopy. Most patient find it uncomfortable having a tube passed through their GIT. I only hope that the shortcomings associated with the devise are taken care of.
Indeed, biomedical engineering and genetic engineering are awesome!
References
•Probiotic cells
•Plant genetic engineering
•Genetically engineered moth
•Genetic engineering
•Recombinant technology
•Ingestible bacteria on a chip
•D.radiodurans
•ingestible pill
Congratulations @cyprianj! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
@tipu curate
Upvoted 👌 (Mana: 5/15 - need recharge?)
Great, thanks for this! Are people already being tested on the incorporation of this chip? Or are only scientists in the process of studying it for further application?
Thanks @carloserp-2000 You know for every discovery ,there are always testing phases. First, the new discovery must be tested on animals before they are used on humans.
So far, the prototype was tested on animal, pig which had a lot of similarity with human system and it worked and yielded positive results.
The final
The next phase is to test it on humans, but the little challenge is the size, which is quite a little big to be ingested for now, currently, work is on going in reducing the size further so that it will be fully applied to clinical use.
For full account and indepth details about the research work, kindly read the article published on sciencemag below
https://science.sciencemag.org/content/360/6391/915
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM. Note that using the steemstem.io app could have yielded an even more important support.