Rutherford: radioactivity and the discovery of the atomic nucleus Part I
Geiger and Marsden went to Rutherford's desk. Ern looked up and asked:
- How was the experiment?
-The alpha particles are being deflected by the sheet and we have verified that 1 out of 8000 particles is bounced, it is deflected with an angle greater than 90º!
-But that's like shooting a shell against a piece of paper and bouncing!
Ernest Rutherford is known mainly for the discovery of the atomic nucleus and for the great figures of physics who studied and worked under his direction. But these facts that would suffice to define a great figure of science, were only a part of his career.
When Ern left New Zealand in 1895 on his way to England, he was 23 years old, three undergraduate degrees under his belt and a deserved fame in the experimentation with electricity. His first destination was the Cavendish laboratory of the University of Cambridge and there he took it under his tutelage J.J. Thomson.
Curiosities of science.
Thomson and Rutherford, electron and nucleus. Thomson discovered the electron, as we discussed in this blog, and took under his tutelage a young Rutherford. But Ern was a restless man and, as soon as he had the opportunity, he emigrated to Canada to obtain a titular position at McGill University in Montreal. However, the discovery of the nucleus did not occur until his return to England, in particular to the University of Manchester.
Rutherford's investigations in Cavendish were initially focused on the detection of electromagnetic waves but, seeing the great value of the New Zealander, Thomson asked him to investigate the propagation of the electric current in the gases, moving him away from works that seemed to lead him towards the creation Of the radio. Rutherford also became interested in radioactivity, very much in vogue after the discoveries of Becquerel and the Curies; this interest would end up giving him great joys.
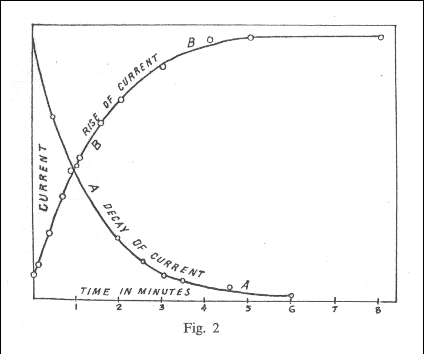
Image source cuantozombi.com
Graphic included by Rutherford in the article in which he described the period of atomic disintegration
Atomic model of Rutherford
The atomic model of Rutherford1 is an atomic model or theory about the internal structure of the atom proposed by the British-New Zealander chemist and physicist Ernest Rutherford2 in 1911, to explain the results of his «gold foil experiment».
Rutherford concluded that the mass of the atom was concentrated in a small region of positive charges that impeded the passage of alpha particles. He suggested a new model in which the atom had a nucleus or center in which the mass and the positive charge are concentrated, and in the extra nuclear zone the negatively charged electrons are found.
Importance of the model and limitations
The importance of Rutherford's model lay in proposing for the first time the existence of a central nucleus in the atom (a term coined by Rutherford himself in 1912, a year after the results of Geiger and Mardsen were officially announced4). What Rutherford considered essential, to explain the experimental results, was "a concentration of charge" in the center of the atom, since, without it, it could not be explained that some particles were bounced in a direction almost opposite to the incident. This was a crucial step in the understanding of the matter, since it implied the existence of an atomic nucleus where all the positive charge and more than 99.9% of the mass were concentrated. Core estimates revealed that the atom for the most part was empty.
Rutherford proposed that electrons would orbit in that empty space around a tiny atomic nucleus, located in the center of the atom. In addition, several new problems were opened that would lead to the discovery of new facts and theories when trying to explain them:
On the one hand, the problem of how a set of positive charges could be held together in such a small volume was raised, a fact that later led to the postulation and discovery of the strong nuclear force, which is one of the four fundamental interactions.
On the other hand there was another difficulty coming from classical electrodynamics that predicts that a charged and accelerated particle, as would be the case of electrons orbiting around the nucleus, would produce electromagnetic radiation, losing energy and finally falling on the nucleus. Newton's laws, together with Maxwell's equations of electromagnetism applied to the Rutherford atom, lead to a time of the order of {\ displaystyle 10 ^ {- 10}} 10 ^ {- 10} s, all the energy of the atom it would have radiated, with the consequent drop of electrons on the nucleus.5 It is, therefore, a physically unstable model, from the point of view of classical physics.
According to Rutherford, the orbits of the electrons are not very well defined and form a complex structure around the nucleus, giving it a somewhat indefinite size and shape. The results of his experiment allowed him to calculate that the atomic radius was ten thousand times greater than the nucleus itself, and consequently, that the interior of an atom is practically empty.
What is radioactivity?
The radioactivity was discovered by the French scientist Antoine Henri Becquerel in 1896 on an almost occasional basis when conducting research on the fluorescence of double uranium and potassium sulfate. He discovered that the uranium spontaneously emitted a mysterious radiation. This property of uranium, then it would be seen that there are other elements that possess it, to emit radiation, without being previously excited, received the name of radioactivity.
The discovery led to a large number of research on the subject. Perhaps the most important in terms of the characterization of other radioactive substances were those made by the couple, also French, Pierre and Marie Curie, who discovered polonium and radium, both in 1898.
The nature of the radiation emitted and the phenomenon of radioactivity were studied in England by Ernest Rutherford, principally, and by Frederick Soddy. As a result it was soon known that the emitted radiation could be of three different classes, which were called alpha, beta and gamma, and that at the end of the process the original radioactive atom had been transformed into an atom of a different nature, that is, a transmutation of an atomic species had taken place in a different one. It is also said (and this is the current terminology) that the radioactive atom has experienced a disintegration.
Radioactivity is a nuclear reaction of "spontaneous decomposition", that is, an unstable nuclide is decomposed into a more stable one, while emitting a "radiation". The daughter nuclide (the one that results from the decay) may not be stable, and then disintegrates into a third one, which may continue the process, until finally a stable nuclide is reached. It is said that the successive nuclides of a set of decays form a radioactive series or radioactive family.
All isotopes of elements with an atomic number greater than or equal to 84 (polonium is the first of them) can be considered to be radioactive (natural radioactivity) but, currently, radioactive isotopes of elements whose isotopes can be obtained in the laboratory natural are stable (artificial radioactivity).
The first laboratory production of a radioactive artificial isotope (that is, the discovery of artificial radioactivity) was carried out in 1934 by the marriage of Fréderic Joliot and Irene Curie, daughter of the Curie couple.
Characteristics of the Model
In 1911, Rutherford introduces the planetary model, which is the most used even today. Consider that the atom is divided into:
· A central nucleus, which contains protons and neutrons (and therefore concentrates all the positive charge and almost all the mass of the atom).
· A crust, formed by electrons, that revolve around the nucleus in circular orbits, similar to how the planets revolve around the Sun.
Rutherford's experiments showed that the nucleus is very small compared to the size of the entire atom: the atom is practically hollow.
### Insufficiencies of the Rutherford model:
1- Contradicted with the laws of electromagnetism of Maxwell, which were widely tested by numerous experimental data. According to Maxwell's laws, an electric charge in motion (such as the electron) should emit energy continuously in the form of radiation, with which a time would come when the electron would fall on the nucleus and matter would be destroyed; This should happen in a very short time.
2- It did not explain the atomic spectra.
References
- Ron, José Manuel Sánchez (November 11, 1993). Space, time and atómos. Relativity and quantum mechanics. AKAL editions. Consulted on December 11, 2015.
- Calahorro, Cristóbal Valenzuela (January 1, 1995). General chemistry. Introduction to Theoretical Chemistry. - - --University of Salamanca. ISBN 9788474817836. Accessed December 11, 2015.
- Landau & Lifshitz, pp. 63-65
-Gribbin, John (2003). «13». History of Science 1543-2001. RBA Collectibles. p. 413 - Bransden, B. H. and C. J. Joachain (1992), Physics of Atoms and Molecules. Harlow-Essex-England, Longman Group Limited. 0-582-44401-2
Bibliography
-Landau & Lifshitz: Mechanics, Ed. Reverté, Barcelona, 1991

✅ @dialexandro, I gave you an upvote on your post! Please give me a follow and I will give you a follow in return and possible future votes!
Thank you in advance!
hello @introduce.bot and I'll follow you I appreciate your support I hope I can count on you to grow on this platform and if you can you make me reestimen this publication thank you greetings.