HESS LAW: What really matters is the start and the end!!!
Hey steemians. So, it’s @eyedeology. I’m usually more into writing abstract. But today I’ve decided to make use of my high school education and do something to create some quality content for the STEM community. My favorite subjects include all of Physics, Chemistry, Biology and Mathematics. Yes, ladies. I’m a nerd. So, you’ll be seeing a lot of it from me soon enough.
Hess law:
In the end, it doesn’t even matter. That’s probably where Linkin’ park was inspired for the song; Hess Law. (RIP Chester Bennington)
Now let us dive into what it is. Hess law, also known as the law of Constant Heat summation, states that, Inspite of the multiple stages of a Chemical reaction, the total enthalpy change in a process is equal to the sum of the changes in each individual stage. This law is based on the fact that Enthalpy is a state function. Now, many of you are wondering what Enthalpy is? And many of you are questioning what a state function is? And some of you might be thinking, why are you here on this planet? I have the question for the first two. Sorry bud, You got to figure the last one out yourself.
Enthalpy?
Enthalpy is a thermodynamic property. A property that describes a system in terms of energy. This means, Enthalpy is a description of a system’s energy. But the energy we are concerned with is heat energy. And Enthalpy is exactly the heat energy of a system at a state of thermodynamic equilibrium.
State function?
And since Enthalpy is described for a particular state at equilibrium, the enthalpy change between two states of a system only depends on the beginning state enthalpy value and the final state enthalpy value and the difference of it. It does not concern with anything in between. That is the definition of a state function. Any function, or variable, whose change only depends on the initial and final value without any concern for the path followed to achieve the final value is called a state function.
Alternatively, a function whose change depends on the path followed is called a Path Function . Rihanna actually has an affinity for path functions since she has dedicated an entire song to one of its prime examples.(a song that took very little of what it is named) Work, for example, is a path function since it depends on the way the state of a system has changed and what means have been used.
And since Enthalpy is a state function, no matter how many stages you’d put a reaction through, the change would ultimately be the same. This is kind of like, the conservation of energy. No, this is an example or more like, the application of conservation of energy.
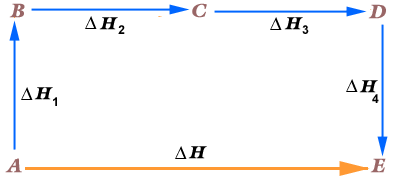
By the power of Hess law vested in me by my high school AP Chemistry education, I shall explain this figure. So, hess law suggests, wait that’s too weak. Hess law say! Yes Hess law states it like a dominant proud correct law that it is, that if a Reactant were to go from A to E, and there was a change in Enthalpy, 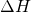
. And then again, we went from A to E, and we made detours to B, C and D respectively with change of Enthalpies, 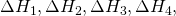
by hess law:
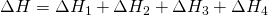
Here is an example of how hess law works:


I hope you’re all familiar with Chemical symbols. So, 2 moles of Carbon(C) burn with one mole of Oxygen(O2) to get oxidized incompletely to give two moles of Carbon Monoxide (CO). And upon further burning with Oxygen, the Carbon Monoxide gets further oxidized to Carbon dioxide. Since, the process was Combustion, there was burning. During burning, Energy is liberated. Why? Because when something burns, you feel hot don’t you? It’s the heat dissipating from the combustion. Such reactions where heat is released during the reaction are called exothermic reactions. (The alternative being called endothermic reactions where heat is absorbed)
So, we’ve established that the two reactions are combustion reactions and hence they are exothermic reactions.
Now let us take a look at this reaction:
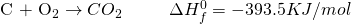
Here, what we had achieved in two different steps has now been achieved in a single step. What? So, why were we doing the two steps? Because we’re trying to prove a point. And you can’t always get what we want. Also, scientists can be very petty at times. Sigh!
Now from the first two equations, it is seen that the change in enthalpy required to form two moles of Carbon Monoxide is -221 KJ/mol. The negative sign indicates that the reaction is actually absorbing heat instead of releasing it.
Therefore, by unitary method, the enthalpy change for one mole formation is -110.5KJ/mol. This number is known as the Enthalpy of formation. It is the amount of heat required to form one mole of any substance.
Similarly, the Enthalpy change for formation of 2 moles of CO2 from CO is -566 KJ/mol. So, for one mole the value is -283KJ/mol.
Now let’s do some Maths.
If we see the amount of enthalpy change in the two steps it is; -110.5 + (-283) = -393.5 KJ/Mol.
Coincidence?? I think NOT!
This is Hess law. All values of the enthalpy are not known to me by some divine nature. There are standard tables where you can find these values which I will link at the end.
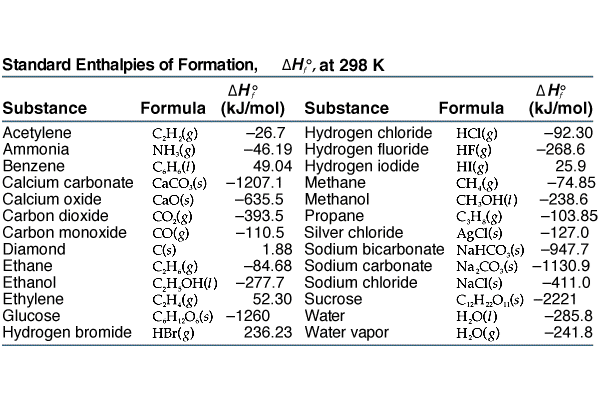
Meanwhile here’s a table for standard values of heat of formation of various molecules. Check it out.
But there is a certain shortcut I have developed, perhaps its documented somewhere, I do not know, which you could use to try and verify Hess Law and perhaps use it to find out enthalpy for various other problems.
Take the first to equations and add them up. Reactants on reactant side, Products on product side and enthalpy on enthalpy side.
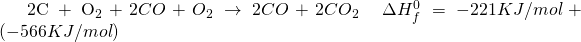
Now treat symbols like algebraic variables and take things around.
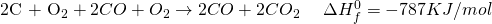
The 2CO on both sides get cancelled and we are left with:
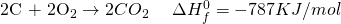
Now for 1 mole of 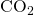 , dividing throughout with 2.
, dividing throughout with 2.
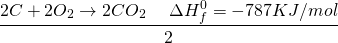
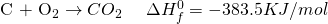
See the miracle of simple algebra? Actually, the process works because the entire thing is a LIE? Not exactly. By manipulating the equation, we are basically doing the same thing as writing a single equation. If you have learned chemistry in high school, you probably know of many examples where two step reactions can be converted into a single step reaction.
And if we go even deeper, we can find out where exactly these enthalpies go to from a molecular level where we can look at the break down of bonds and the energy associated with it. That will be the second part of this post. Stay tuned to hear from me again. Thank you! Peace!
My References:
https://www.masterorganicchemistry.com/2010/09/01/from-gen-chem-to-organic-chem-pt-10-hess-law/
https://www.chemteam.info/Thermochem/StandardEnthalpyFormation.html
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch21/chemical.php
SUCHOCKI, J. A. (2018). CONCEPTUAL CHEMISTRY. S.l.: PEARSON.
I cannot end this post without thanking my friends, @singhbinod08 for suggesting me to do something with my science education and @bikkichhantyal for helping me with coding the equations. Thank you guys! This has been possible because of you.
You got a 0.78% upvote from @postpromoter courtesy of @eyedeology!
Want to promote your posts too? Check out the Steem Bot Tracker website for more info. If you would like to support the development of @postpromoter and the bot tracker please vote for @yabapmatt for witness!