Analysis of the synthesis of CNTs by arc discharge
Analysis of the synthesis of CNTs by arc discharge.
Hello science lovers, today I want to continue with the explanations that I have been giving to you on nanomaterials; more specifically on the synthesis of nanomaterials, whose processes are of fundamental importance for the creation of new technologies that allow better production methods.
The Arc Discharge method.
The Arc Discharge method was initially used to manufacture fullerenes rather than to obtain CNTs. However, in recent years it has been retouched to obtain large-scale CNTs.
The arc discharge process is basically defined as the vaporization of two electrode material by means of the concentration of a potential difference between both electrodes.

Source
CC BY 2.5
The system.
The system consists of a pair of graphite electrodes which are supplied with a voltage that can be direct current, alternating current or in remote cases radio frequency. Some type of metal must be positioned in one of the electrodes, which will assume the function of being catalysts in the synthesis process. The presence of metal is indispensable because without its presence it's not possible to obtain CNTs.
Source
CC BY-SA 3.0
The metals typically used are Fe, Co & Ni.
 Image made by me
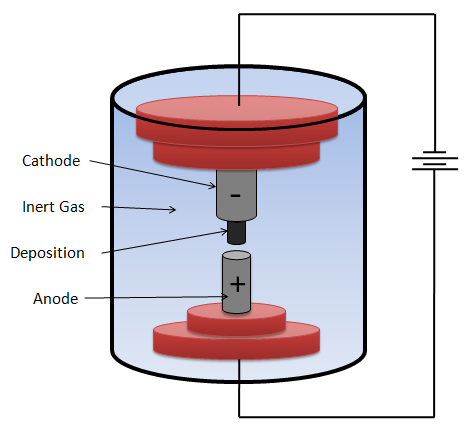
Image made by me The metals are usually inside an opening arranged in the anode or the anode can be doped with any of these metals. The electrodes are located at a very short distance and immersed in an inert or vacuum atmosphere so that the synthesis can be executed.
To achieve these conditions of purity and pressure, the chamber consists of a gas inlet and an evacuation system.
Source
CC BY 3.0
The process.
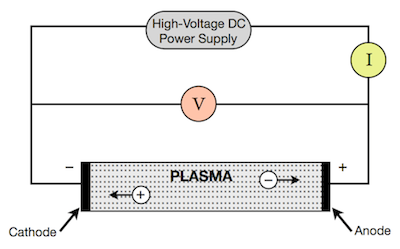
The process starts with the deposition of the system through a vacuum pump, until the necessary vacuum is reached. With the output of the inert gas and the vacuum pump, the pressure inside the system that is regularly around 500 Torr can be controlled. After that, a voltage of approximately 20V, with currents between 50 to 100A, is applied to the electrodes and the evaporation process of the carbon electrodes begins.
Source
CC BY-SA 3.0
The application of current between the electrodes produces several discharges in the space between them. These discharges increase the surface temperature of the electrodes and the carbon and metal atoms are evaporated. As a consequence, the metal atoms are stacked resulting in the formation of metal nanoparticles. The carbon structures lower their temperature and condense in the walls of the chamber to result in the generation of carbon nanoparticles, amorphous carbon and in smaller amounts CNTs.
It should be noted that a larger portion of these semi-stable structures formed on the surface of the metal nanoparticles is deposited at the cathode in a gradual manner, resulting in the formation of CNTs.
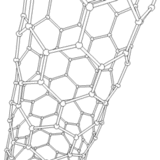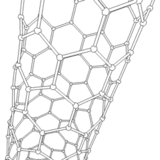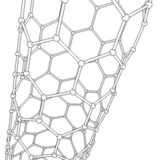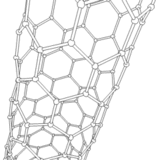
Source
CC BY-SA 3.0
Finally, the application of current to the electrodes is stopped, the chamber is opened slowly to let air inside and finally the cathode is disengaged.
Obtaining SWCNTs by DA.
It is feasible to produce SWCNT through the arc discharge, but it requires mixing metal catalysts, such as Fe: Co or Ni: Y, intrinsically at the anode. In addition, a larger set of parameters have been inspected, including higher vacuum pressures in the system and the use of an alternating current.
Different characteristics according to the operating parameters
The variation of the parameters affects the final characteristics of the CNT:
If the process occurs in a gas atmosphere of He, a large amount of MWCNT is achieved in comparison with the number of impurities.
If methane gas (CH4) is used, MWCNT with high crystallinity and a relatively low impurity rate are generated.
If a Ni-Co alloy is used as a catalyst, in addition to changing the voltage source from an alternating current source, SWCNT with a high production rate is produced.
References.
If you want to read more scientific articles of good quality, do not waste your time, and visit the hashtag #steemstem.
Disclaimer: All the images used are correctly labeled for reuse.

Being A SteemStem Member
Your Post Has Been Featured on @Resteemable!
Feature any Steemit post using resteemit.com!
How It Works:
1. Take Any Steemit URL
2. Erase
https://3. Type
reGet Featured Instantly � Featured Posts are voted every 2.4hrs
Join the Curation Team Here | Vote Resteemable for Witness
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by giovaabbatichio from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.