John Dalton "The father of atomic theory"
Hello my dear Steemians, today I want to talk to you about a person who maybe some when reading the title of the publication noticed something familiar in his surname "Dalton"; They may not know it, but they are sure that they know what "Daltonism" is; condition that at that time had no name but discovered.
I want you to know more about this British who knew how to combine different areas of science and deliver great scientific contributions. On September 6, 1766, John Dalton was born in England, a scientist who changed our understanding of the subject forever thanks to his theory that explains the behavior of atoms.
A rather precocious, characteristic genius that would accompany the rest of his life. Recognized as a scientist and with a solid academic position, Dalton discovered the so-called law of multiple proportions, which governs the weight of the elements involved in a chemical reaction, and proposed as an interpretation of it a whole theory about the constitution of the matter that resumed Greek atomism: it is the so-called atomic model of Dalton, which, in force throughout the nineteenth century, would allow the impressive advances recorded by chemistry during this period. In this sense, the contribution of Dalton has a transcendence almost comparable to that of the «father of chemistry», Antoine Lavoisier, who had laid the foundations and methods of the new science at the end of the last century.
Biography
Dalton's interest extended to pneumatics, astronomy and geography, and in 1787 he began to earn extraordinary income by giving lectures. He also went to a nearby museum with an offer to sell the eleven classified volumes of his botanical collection. He collected butterflies and studied snails, ticks and worms; He also measured his food intake and compared it with waste produced by the body. At the same time, he was preparing to enter medical school, but his family discouraged him from lack of money and confidence in him.
At the age of 26, Dalton discovered that neither he nor his brother could distinguish the colors. He gave his mother stockings (which he thought were blue) and she asked in surprise why he had given her purple stockings, a color that was not appropriate for a Quaker woman. Two years later, in his first important scientific article, Extraordinary Facts Related to the Vision of Colors (1794), John Dalton would provide a scientific description of this phenomenon, which would later be known as color Daltonism.
A year earlier, in 1793, Dalton had published his first book, Observations and meteorological essays, where he defended the thesis that air is not a chemical combination, but a purely physical mixture of gases. That same year he moved to Manchester as tutor and professor of physics and mathematics at the New College of this city, founded by the Presbyterians, and whose prestige rivaled that of the universities of Oxford and Cambridge. He immediately enrolled in the Manchester Library and in the Literary and Philosophical Society, of which he would become secretary and president.
Professor and researcher
These two scientific works had given him some notoriety, and, already with a more comfortable economic situation, he could alternate teaching with research in the laboratory. In 1802, in the memory titled Absorption of gases by water and other liquids, he established his law of partial pressures (Dalton's Law), according to which the pressure of a mixture of gases is equal to the sum of the pressures of each component. He also established a relationship between vapor pressure and temperature. His interest in gases was derived from his love of meteorological studies: he always carried his time devices with him wherever he went, making more than two hundred thousand observations in his diary throughout his life. Thanks to these observations, his analytical mind was able to find numerical relationships between the data.
In 1803 he began to make his greatest contribution to science. He was studying the reaction of nitric oxide with oxygen when he discovered that the reaction could take place in two different proportions: sometimes 1: 1.7 and others 1, 3.4 (in weight). This led Dalton to establish the law of multiple proportions, according to which, in a chemical reaction, the weights of two elements always combine with each other in proportions of small integers; looking for an interpretation of this phenomenon, he began to outline the principles of his atomic theory.
The results were communicated orally that same year and published in 1808 in a book that is his most famous work: New system of chemical philosophy. In it he adopted the notion of atom and established the postulates of the constitutive theory of matter that we now know as Dalton's atomic theory; he drew individual particles to illustrate chemical reactions and published his first list of weights and atomic symbols.
Not everyone accepted the new theory; in 1810 he published the second part of the New System of Chemical Philosophy, providing new empirical evidence. The third part would see the light in 1827. Although he was a member of the Royal Society since 1822 and in 1825 he received the medal of this scientific society for his work in atomic theory, Dalton always considered himself as a teacher and won his life.
The atomic theory of Dalton
The concept of the atom goes back to the debates between Greek philosophers developed from the sixth century BC One of the questions that most occupied these thinkers was the determination of a constitutive and original principle (arjé or arche) common to all beings; thus, for Thales of Miletus, all things and also living beings are composed of water, from which they originated by processes of evaporation and condensation. Other philosophers suggested other substances as a common substrate (for example, air according to Anaximenes) or principles of a more abstract nature.
In its fruitful development, the arche question would lead the philosophers Leucippus and Democritus to formulate the atomistic doctrine, according to which all beings are composed of a plurality of particles of imperceptible magnitude, atoms (in Greek, atoms means "indivisible") . ); the combination of atoms of different sizes and shapes in different orders and positions explains the diversity of beings we observe in the physical world, although all of them are made up of atoms. The atomistic doctrine, however, never came to be considered as a confirmatory scientific hypothesis, but as a philosophical speculation about the attempt to reconcile the theses opposed to those that Parmenides and Heraclitus had arrived at. For more than twenty centuries, atomism was archived as something of secondary interest among scientists, until the idea was reborn in the first decade of the nineteenth century, by the hand of John Dalton.
John Dalton had not intended to formulate a theory about the constitution of matter; he came to it as a result of his research on gases, and his goal was none other than to explain the discoveries made in them. In his memory Absorption of gases by water and other liquids (1802), he had established his well-known law of partial pressures: the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each of its components.
A natural continuation of these studies was to investigate the composition of the same gases (and especially oxides of nitrogen, oxygenated compounds of sulfur and carbon, methane, etc.). Repeated experiences would lead him to discover the law of multiple proportions: if in a chemical reaction two or more elements are combined and the weight of one of them is kept constant, the weight of others varies according to simple relations expressible in whole multiples. In other words, substances always react with others keeping a constant relationship between their respective weights; they can be combined in large or small quantities, but that same proportion is always maintained.
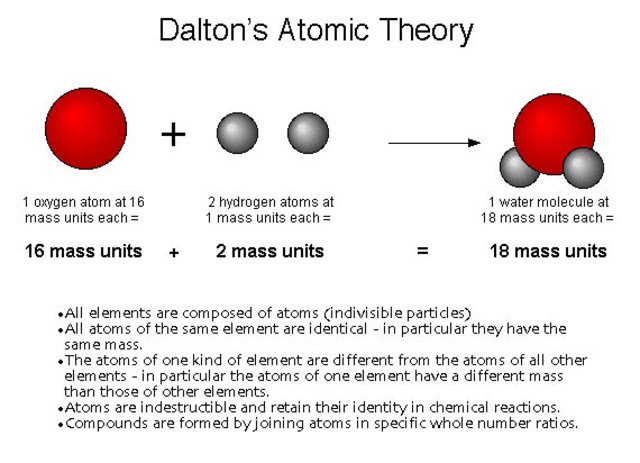
To explain these arithmetical relations, John Dalton supposed that every element should be made up of concrete amounts of matter, which made the existence of multiples of them comprehensible and explained that only certain values of their weights intervened in a reaction. He returned to the atomic theory of Democritus, which considered matter formed by indivisible particles. The existence of interatomic spaces, on the other hand, justified the compressibility of the gases, the changes of state and the phenomenon of dilation, inexplicable facts if the discontinuity of the matter was not taken into account.
On October 21, 1803, Dalton presented his atomic theory for the first time at a conference he gave in Manchester, organized by the Literary and Philosophical Society, before an audience of seven people. No wonder the lack of public, because Dalton had no reputation as a good speaker. But his theory got more disclosure to publish, in 1808, in the first part of his work New system of chemical philosophy.
The atomic theory of Dalton established a series of fundamental postulates: the elements are formed by atoms, tiny material particles that can not be created, destroyed or divided; all the atoms of a certain element are identical, both in the mass and in its other properties; the atoms combine with each other in simple proportions, expressible in whole numbers, to form "compound atoms" (what we now call molecules, a concept that would be introduced by Amadeo Avogadro); all the "compound atoms" of the same substance are identical, both in the mass and in its other properties.
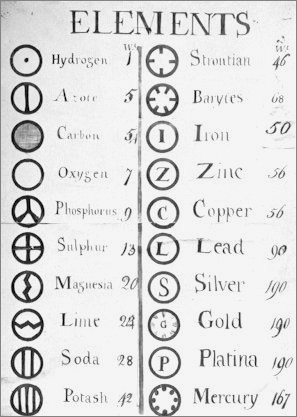
The law of multiple proportions and the atomic hypothesis led Dalton himself to the first attempt to make an instrument of fundamental utility for chemistry: a table of atomic masses, which appeared in the first part of the New System of Chemical Philosophy. Dalton chose hydrogen as the pattern for the atomic mass table and gave the atom of that element a mass of 1. Naturally, he could have chosen any other element and any other value for its atomic mass, but hydrogen was the lightest of the atoms elements and 1 is the number that allows comparisons to be made more easily.
The table that Dalton elaborated was incorrect for two main reasons: first, he did not know the correct relation of combination of the atoms in a chemical reaction, and secondly, the equipment used at the time to determine mass relations was not very precise . As a result, the established values were significantly lower than the real ones. However, his table was an important first step in the determination of the atomic masses, and just twenty years later, the Swedish chemist Jöns Jacob Berzelius was already able to establish a list of atomic masses with values very similar to those currently accepted.
Considered one of the foundations of modern science, Dalton's atomic theory would be revealed as an extraordinarily fertile hypothesis for both chemistry and physics, and it remained valid for almost a century. It was necessary to wait for the discovery of subatomic particles (which ended the dogma of the indivisibility of the atom) to see substantial changes in the model, reflected in the successively improved atomic theories of Joseph John Thomson, Ernest Rutherford, Niels Bohr and Arnold Sommerfeld, already at the beginning of the 20th century.
On July 27, 1844, he died of a heart attack. According to his wish, after his death he underwent an autopsy to determine the cause of what would later be called color blindness. He demonstrated that color blindness is not an eye problem, but was caused by some deficiency of sensory power. He was buried with the honors of a monarch, at a funeral followed by more than four hundred thousand people, in contravention of the principles of the Quakers according to which he had lived.

More Information:





Nice post @jennifer.jimenez 0_0
Thanks eddith (=
You got a 9.40% upvote from @ipromote courtesy of @jennifer.jimenez!
If you believe this post is spam or abuse, please report it to our Discord #abuse channel.
If you want to support our Curation Digest or our Spam & Abuse prevention efforts, please vote @themarkymark as witness.
You got a 13.36% upvote from @bid4joy courtesy of @jennifer.jimenez!