Electrostatic: Coulomb's Law
HELLO STEMIANS!
Electrostatics studies the mutual effects that occur between bodies as a consequence of their electrical charge. The electric charge is the quantity that quantifies the electrostatic phenomena. The elementary charge is that of the electron, equal and opposite to that of the proton. Normally multiples of this charge is used, such as columbium.
Source
Coulomb's law
The electrostatic interaction between charges can be expressed mathematically by the so-called Coulomb Law, established by the French physicist Charles A. Coulomb in 1977:
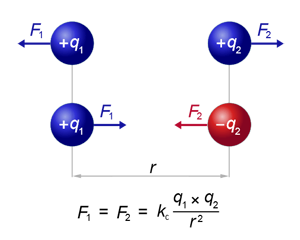
The force exerted between two point electric charges q1 and q2 is directly proportional to the product of both charges and inversely proportional to the square of the distance that separates them.
Where the unitary vector of direction is the line that joins the charge q1 with the charge q2 and its sense that goes from q1 to q2. K is the so-called Coulomb constant, which in vacuum takes a value 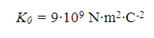
Source
Note that it is a vector magnitude, and as such it will be characterized by:
• Module: The numerical value of F.
• Direction: The one of the line that unites both charges.
• Sense: If both charges are of the same sign, the force will be positive (repulsive) while if they are of opposite sign the force is negative (attractive) as observed experimentally.
Another characteristic to keep in mind is that because it is a force must comply with the third law of Newton (Principle of action and reaction) and therefore the forces that are exerted mutually two charges must be equal in module and direction but in the opposite direction (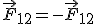 )
)
Keep in mind that this law is valid only for point loads and at rest, although, as a valid approach, it can also be used for loads whose size is much smaller than the distance between them and when the movement of the loads occurs at very low speed or in uniform rectilinear trajectories. That is why it is called electrostatic force.
In the following animation you can observe the vectorial character and the dependence with the square of the distance of the electrostatic interaction, as well as the attractive or repulsive nature of the force depending on the sign of the charge, as indicated by Coulomb's Law. To do this, move any of the charges and observe the change in force vectors.
It is important to note the following points in relation to Coulomb's law:
a) when we talk about the force between electric charges we are always assuming that they are at rest (hence the name Electrostatics);
Note that the electric force is a vector quantity; it has magnitude, direction and sense.
b) the electrostatic forces fulfill Newton's third law (law of action and reaction); that is, the forces that two point electric charges exert on each other are equal in module and direction, but in the opposite direction: Fq 1 → q 2 = −Fq 2 → q 1 ;
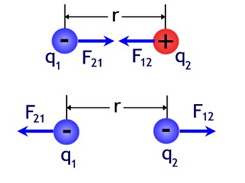
Source
In mathematical terms, this law refers to the magnitude F of the force that each of the two point charges q 1 and q 2 exerts on the other separated by a distance r and is expressed in the form of an equation such as:
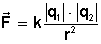
k is a constant known as Coulomb constant and bars denote absolute value.
F is the vector Force that electric charges suffer. It can be attraction or repulsion, depending on the sign that appears (depending on whether the charges are positive or negative).
- If the charges are of opposite sign (+ and -), the force "F" will be negative, which indicates attraction
- If the charges are of the same sign (- and - or + and +), the force "F" will be positive, indicating repulsion.
In the graph we see that, independent of the sign that they have, the forces are always exerted in the same direction (parallel to the line that represents r), they always have the same module or value (q1 x q2 = q2 x q1) and they are always exercised in the opposite direction between them.
Recall that the unit by electric charge in the International System (SI) is the Coulomb.
c) As far as we know Coulomb's law is valid from distances of many kilometers to distances as small as those between protons and electrons in an atom.
Having understood Coulomb's law by its concept, we can determine the application of this law in everyday life.
1- The first example deals with when after handling a polyethylene bag and this has manufacturing residues, we must fight enough to detach them from our body, since we are charged by the friction of clothes, air, etc. These charges are opposite to those of plastic and are attracted to us; therefore they stick to our clothes, hands, body, etc.
2- Another example very similar to the previous one is to rub two balloons, when approaching them at a certain distance they attract each other.
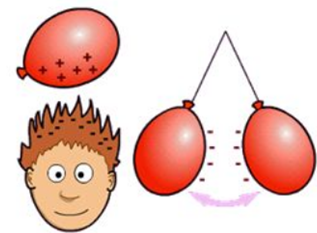
Source
For mor information, visit:
- https://physics.info/coulomb/
- http://www.chegg.com/homework-help/definitions/coulombs-law-4
- http://science.jrank.org/pages/1838/Coulomb.html
Thank you for u attention!
Source: http://e-ducativa.catedu.es/44700165/aula/archivos/repositorio/3000/3229/html/2_interaccin_electrosttica_ley_de_coulomb.html
Copying/Pasting full texts without adding anything original is frowned upon by the community.
Some tips to share content and add value:
Repeated copy/paste posts could be considered spam. Spam is discouraged by the community, and may result in action from the cheetah bot.
Creative Commons: If you are posting content under a Creative Commons license, please attribute and link according to the specific license. If you are posting content under CC0 or Public Domain please consider noting that at the end of your post.
If you are actually the original author, please do reply to let us know!
Thank You!
One or more of your photos is breaking some form of copyright, whether it be that the photos require proper attribution or are licensed in such a way that they are not free to use. For more information, check out this post here on steemstem copyright standards.
Sincerely,
@kryzsec
Congratulations @kowalskii! You received a personal award!
Click here to view your Board
Congratulations @kowalskii! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!