A Day with Microbiologist: Isolation and Purification of the Small Peptides Inhabiting the Antimicrobial Properties
Hello Everyone,
Microbial invasion to our world has cost us a lot and its threat is gaining the height day by day and to overcome these microbial invasion we all are trying our best. So many new techniques and methods have been applied to overcome this issue. With all this Anti-Microbial Peptides (AMP) are also a class of antibiotics or antimicrobial molecule which is in fashion.
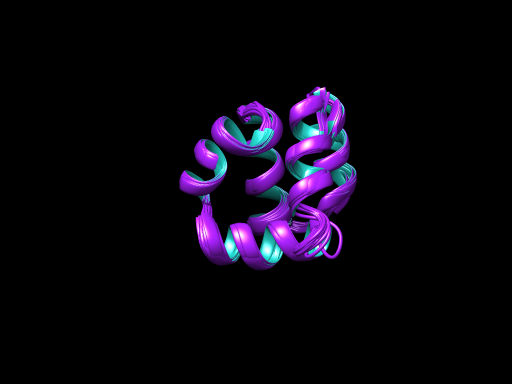
PDB 1ZNI Structure made in UCSF Chimera 1.10.1
Antimicrobial peptides are proteinaceous defense molecule produced by almost every class of organism and are a part of innate immune response. Plants are known to produce these AMPs in a wide variety and in huge amount for the defense against the insects and all. There are other eukaryotes also which are known to produce such peptides to overcome the microbial infection, we will be discussing it some other time. While prokaryotes produced these AMPs to sustain in their population. Down the ground in the microbial world there is huge fight for existence is going on and the fittest will survive. Microorganism produce these peptides to inhibit the growth of other species so that their own generation can flourish. Class of these peptide is huge and are divided on the basis of their charge and structure they attain on proper folding (1).
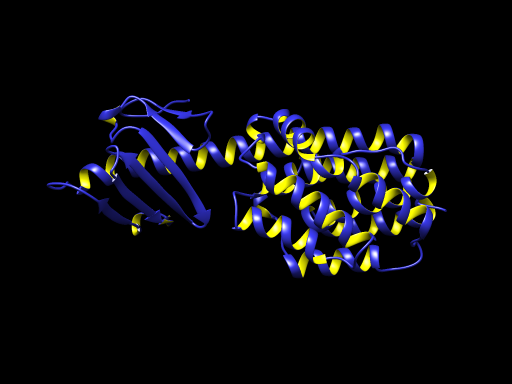
 PDB 1E68 Structure made in UCSF Chimera 1.10.1
PDB 1E68 Structure made in UCSF Chimera 1.10.1Bacteriocine
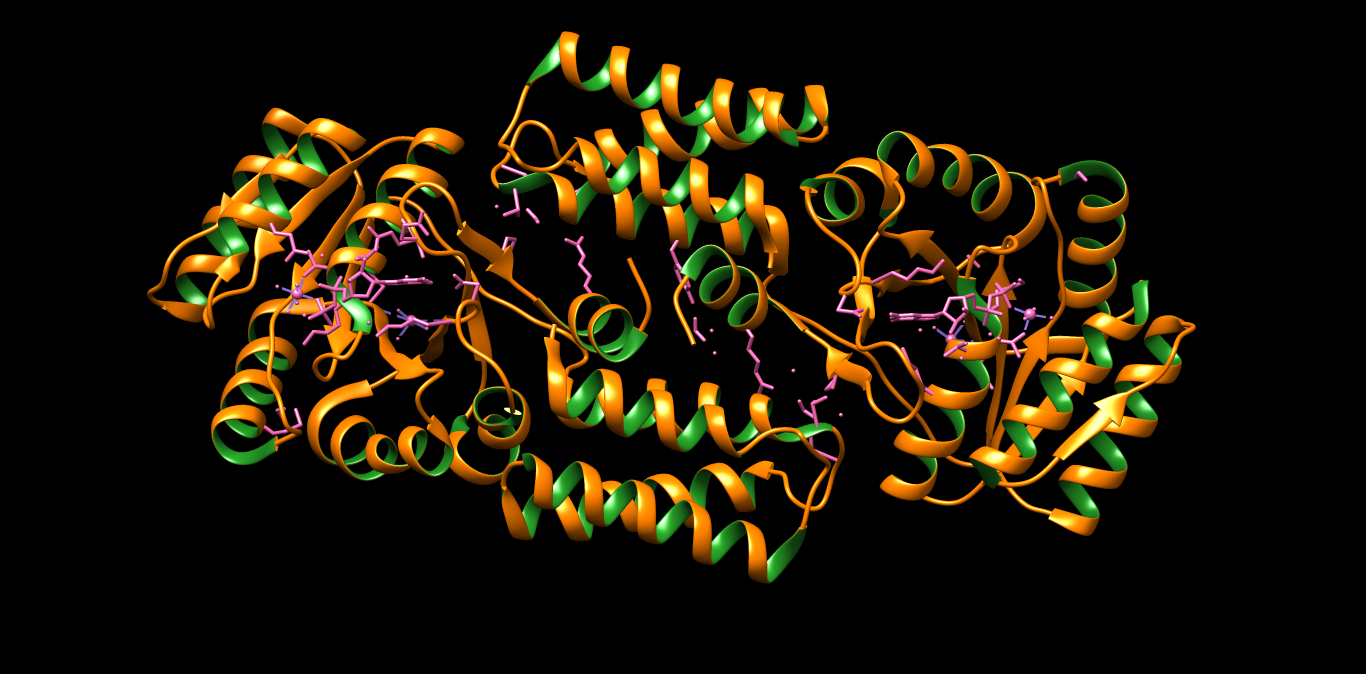
 PDB 1A87 Structure made in UCSF Chimera 1.10.1
PDB 1A87 Structure made in UCSF Chimera 1.10.1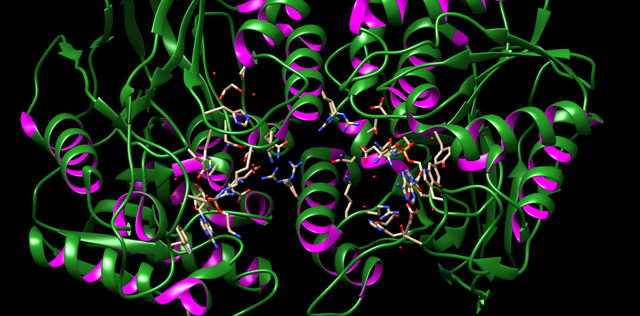 PDB 3TLC Structure made in UCSF Chimera 1.10.1
PDB 3TLC Structure made in UCSF Chimera 1.10.1Bacteriocine from Gram Positive – They are subdivided into four class of bacteriocins; Class I which deals with the small sized peptide below 5KDa example: nisin. Class II contains peptide below 10KDa which have N-terminal consensus sequence and C-terminal is causing the cell membrane permeability in the target cell. Class III are also known as bacteriolysin and functions by cell lysis and are more than 10KDa in size. Class IV have some sugar group attached to the protein part and enhancing the killing activity of the peptide (8).
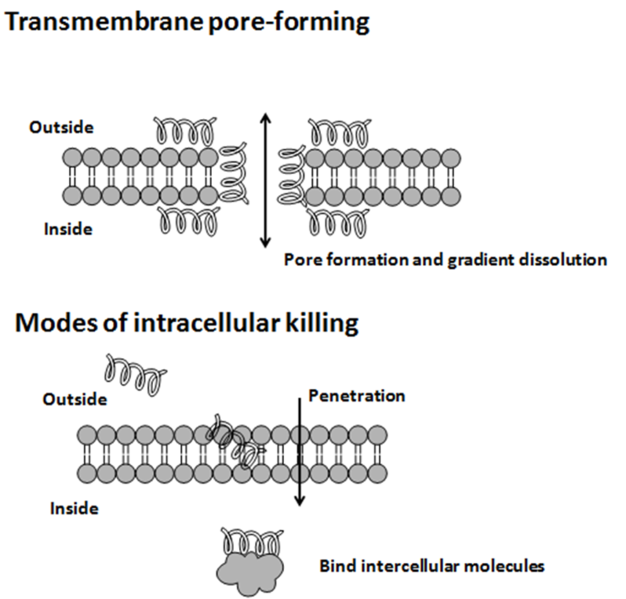
Why and when Bacteria produces it?
You must have guessed the answer of this, bacteria produces these peptide in the stationary phase of their growth. The time when the nutrient start to get finish and the population of bacterial cells are huge and all of them wants the food at that time bacteria secrets such peptide to kill the weak ones that’s why they are also called as secondary metabolites.
How it can be observed?
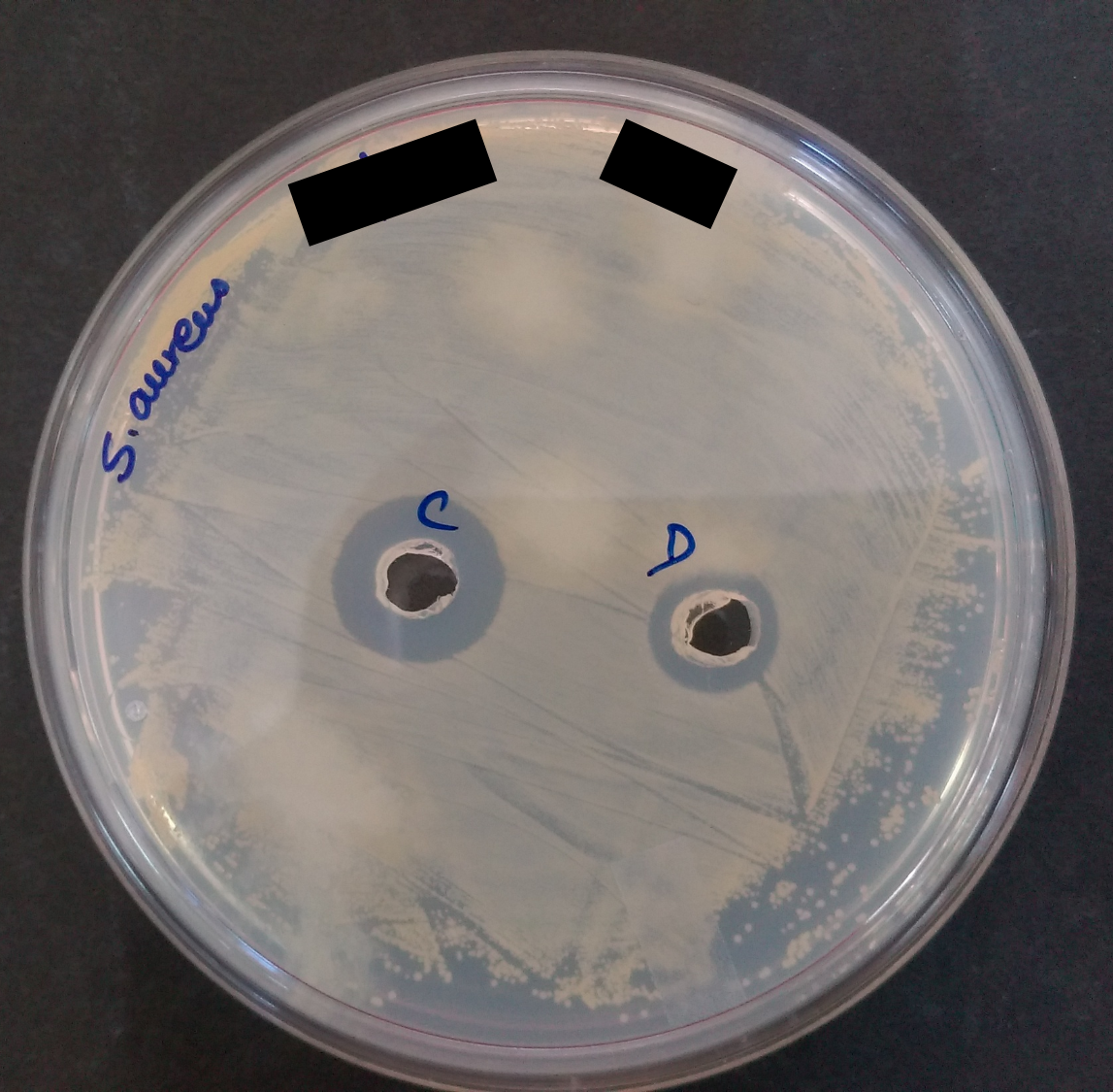 Well Assay showing the zone of inhibition
Well Assay showing the zone of inhibitionThe whole process of its separation incudes multiple techniques: the first step would be to grow the bacterial culture in bulk and as these AMPs are produced as extracellular proteins can be extracted from the supernatant of bacterial culture. Building of huge amount of froth in the media is a sign of production of extracellular proteins. Through centrifugation these peptides present in the supernatant can be extracted. Next step would be, the crude extraction procedure which include the use of some silica based resins which have the affinity for binding to the hydrophobic molecule and rotating the supernatant with these resin for few hours can separate almost all the present peptides in the supernatant to the resins and by washing the resin again with the methanol can give you the crude extract of the AMP from your bacterial strain. As it is in the crude form and further needs more purification for use.
Determining the Minimum Inhibitory Concentration (MIC)
From the crude extract only, the MIC can be generated this gives the idea about the concentration needed to inhibit the bacterial population. It was performed by subjecting the test strain with the extracted peptide in a gradient concentration starting from the lowest to higher. The concentration which start showing the bacterial inhibition is considered as the MIC and suggests the minimum concentration required to kill the specific bacterial species (9). This also gives an idea about the protein concentration and the efficiency of the peptide.

96 well plate showing the different growth based on concentration of AMPsWikimedia Commons
Purification of the Extracted peptide
The extracted peptide was then purified through different chromatographic method, all of these are dependent on the charge of the peptide and the size of the peptide. Gel-filtration column chromatography with different silica resins can be used to purify these peptide Sepadex G50, G100 G150 are the common ones. When the peptide contains some lipid part, the purification protocol changes totally as the lipid containing parts responsible for the antimicrobial activity would be coming near the size of 1-2KDa so it will be very much difficult to isolate it through the same column. Hence, acid precipitation could be performed to isolate such peptide. Ammonium precipitation a salting out process can also be used to purify protein from such crude extract. If anyone wants, I can share the whole protocol for all these process for the purification of these small peptide.
All the collected fractions from the column was then checked for the activity and on confirmation was pooled in a single container and was further subjected to High Pressure Liquid Chromatography (HPLC) and Mass- Spectrophotometry for the size determination. Preparative HPLC can be used to purify the whole bunch of isolated protein, which will give you the extra purified protein.

HPLC system for the purification of the extracted Antimicrobial Peptides
I know this experimentation part could be little boring but next time on continuing to the same topic I’ll talk about the fascinating side of these peptide which might find interesting to you all.
Images which are not source are taken by me and do not violates any copyright.
For most of the terms I have hyperlinked with the simple Wikipedia page, that will make you understand if not feel free to ask anything below in the comment section. I will be happy to have them and will try to resolve doubts if anyone have.
References
Bahar et. al., 2013 Antimicrobial Peptides. 6(12);1543-1575.
Ulmschneider et. al., 2017 Biophys J. 113(1);73-81.
Yeaman et. al., 2003 Pharmacological Review 55(1);27-55.
Leontiadou et. al., 2006 J. Am. Chem. Soc., 128(37);12156-12161.
Yang et. al., 2014 Front Microbiol. 5:241.
Bierbaum et. al., 2009 Curr Pharm Biotechnol. 10(1);2-18.
Lazdunski et. al., 1998 J Bacteriol. 180(19);4993-5002.
Hassan et. al., 2011 Adv Pharm Bull. 1(2);75-79.

Hi @micro24
We have selected your post as post of the day for our DaVinci Times. Our goal is to help the scientific community of Steemit, and even if our vote is still small we hope to grow in quickly! You will soon receive our sincere upvote! If you are interested in science follow us sto learn more about our project.
Immagine CC0 Creative Commons, si ringrazia @mrazura per il logo ITASTEM.
CLICK HERE AND VOTE FOR DAVINCI.WITNESS
Keep in mind that for organizational reasons it’s necessary to use the “steemstem” and “davinci-times” tags to be voted again.
Greetings from @davinci.witness and the itaSTEM team.
Thankyou @davinci-times team for selecting my post.
Hi @micro24
Nice work. I remember doing those well assays (though I called them spot on lawn assays) and MIC determinations with a Biocycler (at least I think that's what it was called). Either way, its nice to see scientists explain their work :)
Maybe we should CRISPR-Cas9 ourselves some froggy anti-microbial resistance! :D
Last year it was reported that microbiologists at a medical school in Norfolk, Virginia saved a man who was terminally ill with a serious infection. No anti-bacterial medicine was affective. As a last resort, large amounts vitamin C (ascorbate) was delivered into him and the man was out of the hospital within a week.
Can oxidative stress cause us that much harm? I hope you address this in your future post.
Thanks @quiplet
I don't think there is any relation between the peptides synthesized for the defense with the oxidative stress and if there is any I'm not sure of that.