Thermodynamics and Its "Zeroth" Law, will open your mind.
Hello Steemians :)
Have you ever wondered why when you walk into a pool that has cold water, after a while you feel nothing, as if it had been heated? |  |
|---|---|
Do you know why when we have a fever and use a glass thermometer, we must first reduce the temperature and then to measure it? | 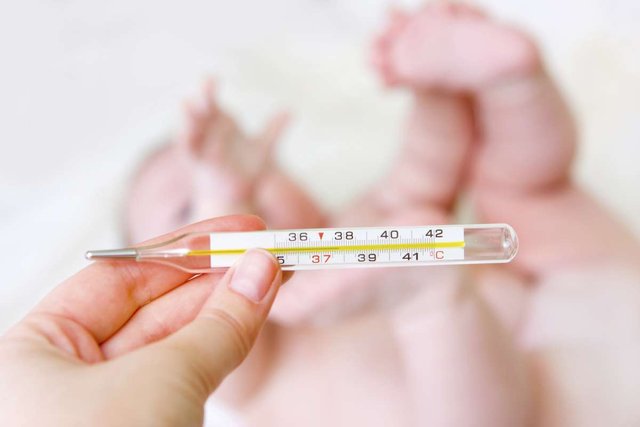 |
The answers to these questions and another ones, you’ll know after reading this post.
First, I will talk about the macroscopic so that we go deeper into the subject, starting with the definition of “Thermodynamics ":
“Thermodynamics is the branch of physics that describes the equilibrium states at the macroscopic level”.
Also, the Dictionary.com website defines the “ thermodynamics” term as follows:
“The science concerned with the relations between heat and mechanical energy or work, and the conversion of one into the other: modern thermodynamics deals with the properties of systems for the description of which temperature is a necessary coordinate”.
In other words, it studies the flow of energy (any type of energy that a body possesses) and the states of equilibrium between several of them. Now, understanding the above definition, we should understand another term or thermodynamic property, the “Temperature”.
Now, I want you to take a minute before reading the definition and think for a moment: What is temperature for you? You know it exists, but you do not know its definition, right?
Here its meaning:
“Temperature is a physical quantity expressing hot and cold. It is a proportional measure of the average translational kinetic energy of the random motions of the constituent microscopic particles in a system (such as electrons, atoms, and molecules)”.
Definition that, although it sounds a little tangled, could be summarized: The temperature comes directly related to the movement of the particles of an object, that is, when something is heated (solid, liquid or gas) its atoms or molecules move more quickly.
The Zeroth Law of Thermodynamics may be stated in the following form:
“If two systems are both in Thermal Equilibrium with a third system then they are in Thermal Equilibrium with each other”.
This law of thermodynamics has been used in devices such as the thermometer to measure temperature. Although the thermometer is primitively used since the time of Galileo, this law was enunciated much later, by James Clerk Maxwell, and formulated as a law later by Ralph Fowler.
 | 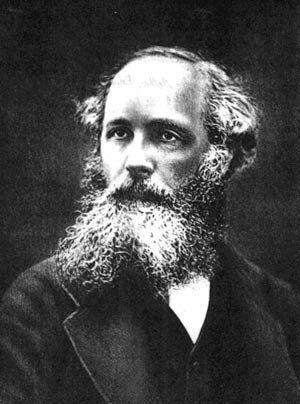 |  |
Galileo Galilei | James Clerk Maxwell | Ralph Fowler |
Now, you have to pay attention to the word Thermal Equilibrium because you have to know the meaning of this word to understand this law:
“Two physical systems are in thermal equilibrium if no heat flows between them when they are connected by a path permeable to heat”.
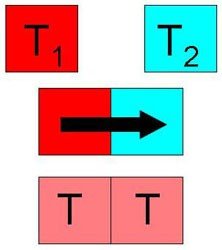
NOTE: In the image, the thermal equilibrium occurs through contact, but not necessarily have to exist.
Basically, the thermal equilibrium is the temperature equality that two materials have (be in solid, liquid or gaseous state).
First Example that will answer the first question made at the beginning.

We all experienced that strong cold feeling we have when entering a pool (as long as the water is slightly cold) and then we stop feeling it, it is because our body enters into thermal equilibrium with the water, even more when we sink completely, in this case we accelerate the equilibrium process.
Let's continue with the example that will answer the second question.

The application of the glass thermometer; all of us at some point we have measured body temperature with one and it is because mercury varies its volume according to the temperature very easily, hence the saying that it has to be shaken slightly to be able to lower its scale and then be used. At the moment that the doctor places it on you, the process of Thermal Equilibrium begins. After waiting for 5 minutes, the mercury will have expanded and the process will have finished.
Last Example: The Hugs

All of us at some point has been cold and the clothes they wear is not enough to eradicate it, therefore, we ask someone else to embrace them, beginning the process of thermal equilibrium, the person will transmit their heat and the two bodies will seek to be placed at the same temperature.
source images: A B C D E F G H I
- Website: https://en.wikipedia.org/wiki/Thermodynamics
- Website: http://www.dictionary.com/browse/thermodynamics?s=t
- Website: https://en.wikipedia.org/wiki/Temperature
- Website: https://en.wikipedia.org/wiki/Thermal_equilibrium
- Book: Thermodynamics: An Engineering Approach by Yunus A. Cengel and Michael A. Boles 6th Edition.
It is a nice article and up-voted it.
But it is important to say that Zeroth law of thermodynamics does not explain why any of these examples (pool, thermometer & hug) happens. It just states conditions of equilibrium.
Explanation of why everything tends to equilibrium is provided by the Second law of thermodynamics:)
Hi @everett57 thanks for your comment, of course, you have the reason, but I preferred to leave it for next posts :) that's because the people who don't know anything about it, they learn easier. Thank you for voting my post and support me, on the next posts, I'll write about the first and second law of thermodynamics :)
Congratulations @natitips! You received a personal award!
Click here to view your Board
Congratulations @natitips! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!