The Leidenfrost Effect
The Leidenfrost effect is a phenomenon of levitation of liquids by its own vapours, observed when a liquid comes into contact with a surface which is a lot hotter than its boiling point. This levitation prevents the liquid from coming into direct contact with the hot surface, causing it to evaporate slower than expected. Each liquid has a corresponding minimum temperature of the surface, called the Leidenfrost point, above which it exhibits the Leidenfrost effect. Before we go ahead, watch this video:
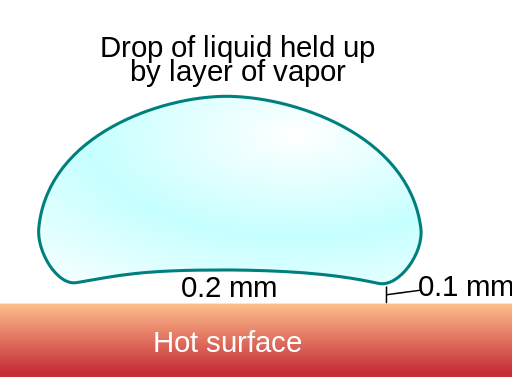
When a liquid comes into contact with a surface hotter than itself, it absorbs heat from the surface and gradually turns into vapour. But when the temperature is too high, the bottom surface of liquid is quickly converted to vapour, but this forms a layer of vapour between the hot surface and the liquid. Since the vapour is usually slower at conducting heat, the bottom surface of the liquid receives heat slower than usual. Therefore it gets converted to vapour slower and the drop of liquid evaporates slower than usual. For example, a drop of water that vaporized almost immediately at 168 °C lasted for 152 seconds at 202 °C.
.png)
By Vystrix Nexoth at the English language Wikipedia, CC BY-SA 3.0, Link
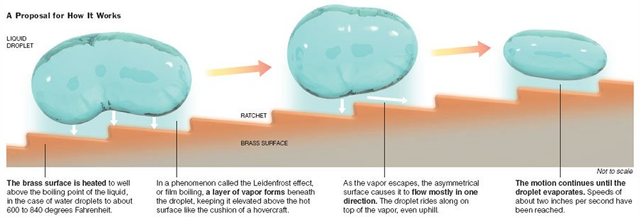 Source: The New York Times
Source: The New York Times
References
- Enhanced Droplet Control by Transition Boiling
- Leidenfrost Effect
- Propulsion on a superhydrophobic ratchet
Follow me @omstavan for more steemSTEM posts!

This banner was made by @nitesh9
So this would be why we can stick our finger in liquid nitrogen for less than a second and emerge unscathed. Thanks for sharing!
Exactly! The same is true why you can quickly dip and remove your wet hand in molten aluminum and nothing will happen. The water turns into vapor and prevents the heat from reaching your skin quickly!
This is awesome. Water is truly a beautiful anomaly.
Very interesting post. Keep up the good work :)
Congratulations @omstavan! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP