 Some contact lenses are made of hydrogels.
Some contact lenses are made of hydrogels.
Image credits
Hello steemians!
This is my first article for the
#steemstem community. In the framework of polymers and biomaterials I will talk about hydrogels. I will profit of my experience and knowledge in this field, as it was part my research project. Briefly, I will define polymers and hydrogels; later, I will mention several and potential applications of this materials, and finally, I will summarise one of the experiments I have done.
Let us start by defining what a "material" means. A material is something related to what we call "matter", and this is everything that surrounds us. According to the Cambridge Dictionary, a material is "a physical substance that things can be made from, for example: building materials, such as stone; and crude oil is used as the raw or basic material for making plastics". There are different types of materials, both natural and synthetic, such as metals, alloys, ceramics, wood, textiles, plastics (composed of polymers), to name the most general.
 Things made of different materials.
Things made of different materials.
Image credits
Materials have been the platform over which all civilizations have growth up. In fact, materials and their applications have been the main inspiration for new branches in Science and for the development of new technologies. The continuous demand for new materials has increased over the time, which has certainly stimulated a huge number of investigations for the creation of more and better ones. It is clear then that, materials Science has had (and still does) an impact in the improvement of existing materials and in the developing of new technologies.
Due to the several advantages of using polymers over conventional raw materials (metals and ceramics), many technological areas, such as medicine, pharmacy, cosmetics, telecommunications, environmental sciences, hygiene, engineering and agriculture, among others, have been supported by polymeric materials. Usually, polymers are are economically accessible, easily processable and chemically modifiable. In addition, polymers can be designed in such a way to having desirable physical and mechanical properties.
What are polymers?
Polymers are macromolecules, that is large molecules, build up of the repeating units called monomers. The reaction that takes place to bind all the monomers is called polymerization. A polymer may consist of hundreds, thousands or larger monomers linked together in a polymeric molecule. To refer to polymers, we need to talk about materials whose molecular weight reach hundreds of thousands or even millions of units. Otherwise we refer to oligomers.
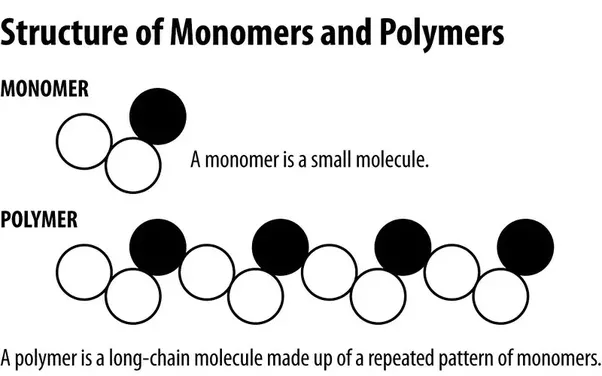 Image credits
Image credits
There is other class of polymers, namely, copolymers, for which the polimerization involves two or more chemically different monomers. Copolymers are classified according to the sequence of the monomers, namely: block, graft and random copolymers. Due to their complexity in composition, copolymers are much more complex than conventional polymers.
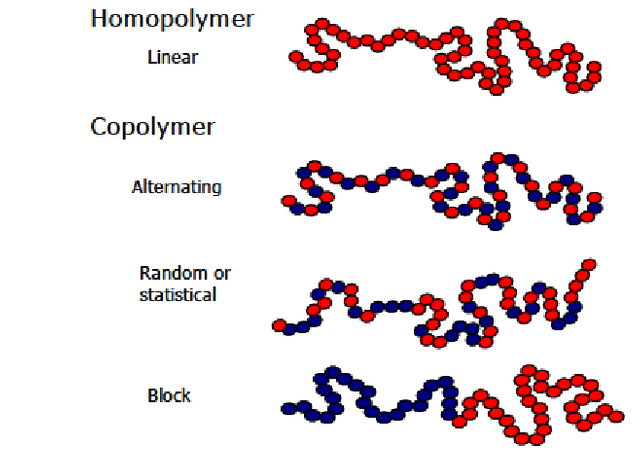
Classification of polymers according to the type of monomer.
Image credits
If no side reactions occur in a polymerization reaction, unbranched or linear polymers are obtained. Under certain experimental conditions, polymers with short or long branches can be synthesized. The final, chemical and mechanical, properties vary considerably depending on the structure, either linear or branched, and on the length of such branches; so chemists play with the reaction variables when searching a given application.
When polymer chains are joined with other neighboring chains of the same or different nature, a three-dimensional network is obtained. The resulting polymer-network, namely a cross-linked polymer, turns insoluble and will not melt. In order to form a cross-linked polymer, it will be necessary for each molecule to join two or more nodes to other molecules. When there are many junctions by primary chain, true cross-linked networks are achieved. So, cross-linked polymers from which three-dimensional networks are built up, are formed by single molecules of infinite size.

Classification of polymers according to the structure.
Image credits
On the other hand, when two chemically different coexisting polymer networks interact, interpenetrated polymer networks (IPN), are formed. They originate when two incompatible polymers intertwine under specific reaction conditions. A source of IPNs is, for instance, the trapping of polymers in the network of different polymers. The combination of polymers also lead to a multi-component polymer matrix with a new behavior. However, that strategy can be used in modifying, the physico-chemical properties of polymer, in such a way of satisfying specific needs required.
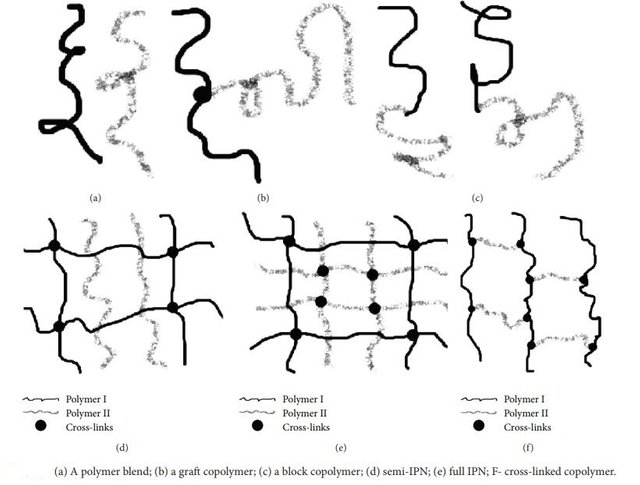
Author: Lohani et al, see reference number 1.
The polymeric biomaterials.
As the title of this post suggest, "biomaterials", are the key in the present topic. A biomaterial will therefore be any substance designed to be in contact with biological systems or living beings.
A material can be used as a biomaterial if it is compatible with the system where it will be introduced. It must possess characteristics similar to that system, so that it is capable of assimilating it. This is where the concept of "biomimicry" appears; that means new technologies are created simulating biological systems, copying nature's way of being

Image credits

Image credits
Currently, the largest class of biomaterials include polymers. They present an extensive number of applications in various scientific and non-scientific areas, such as agriculture, cosmetic, food, medicine and biotechnology industries, to name a few. Regarding these last two areas, its use ranges from the manufacture of scaffolds, artificial organs, prosthetics and orthopedic applications, surgical devices and sutures, bio-aids, drug delivery systems, biosensors, enzyme transporters and cells, up to eye devices, etc.
There are some biomaterials that change their initial forms as response to external stimuli, for which those polymers are considered as 'intelligent'. Such stimuli could be: changes in pH, temperature, increase or decrease in light, presence of electric or magnetic field, being in contact with solutions of high ionic concentration or with biological molecules, etc .

The "smart" cup that smiles and changes color when "feeling" the heat.
Image credits
There are many intelligent polymers. Linear chain polymers, block copolymers, interpenetrated polymer networks, shape memory polymers and crosslinked polymers or gels, stand out in that classification. When talking about gels, the so-called hydrogels, must be considered and that it is what follows:
Hydrogels.
Hydrogels are cross-linked polymeric materials of natural or synthetic origin; structurally they are three-dimensional, and swell upon contact with aqueous solution. As a consequence, soft and elastic materials are formed with a significant amount of water retained in their structure. There are also superabsorbent hydrogels, a different class of hydrogels which can absorb huge amount of water.

Image credits
Hydrogels can be obtained by polymerization and simultaneous crosslinking of one or more polyfunctional monomers. Polyfunctional monomers have at least one of these functional groups: hydroxyl groups (-OH), carboxyl (-COOH), sulfonyl (-SO3H) , amides (-CONH2), among others. These functional groups provide the hydrophilic nature (related to water) to the resulting polymeric material.
Hydrogels absorb water due to the flexibility of their polymer chains. That deform or stretch them, which allow the absorption of water molecules into the three-dimensional structure. In absence of water or aqueous solution, gels are in solid state, being called as xerogels. Once a xerogel comes into contact with water, it absorbs it progressively and in large quantities until it reaches its maximum swelling. In this way, the water is retained in the three-dimensional mesh (imagine a sponge, it is as such but at a molecular level) giving rise to an infinity of characteristic properties. Similarly, there are also hydrogels that swell in the presence of other physical or chemical stimuli, not in vain are in the category of intelligent materials.
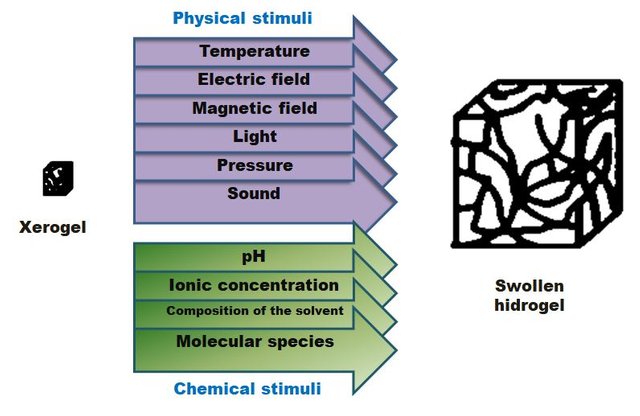
Different stimuli that can swell hydrogels, depending on the characteristics of chemical structure of the polymer(Image of my property.)
Even when hydrogels are hydrophilic, they are insoluble in water. That due to the three-dimensional polymer network. That network consist of nodes or points of union generated by covalent bonds or by physical interactions (hydrogen bridges, of Van der Waals, electrostatic, dipole-dipole, dipole-induced interactions, etc.). Additionally, hydrogels have a soft and elastic consistency which largely depend on the starting monomers and the crosslinking density achieved in the polymerization reaction.
Some applications of hydrogels.
Diapers.
The absorbent polymeric materials were introduced in the diaper industry 3 decades ago. They were commercially produced, as feminine sanitary napkins, for the first time in Japan in 1978 . The use of superabsorbent hydrogels has made diapers increasingly thinner and lighter, which implies a reduction of costs in the inherent industrial processes and a lower environmental impact. In that case, a gel is placed in the diaper system, which, absorb the urine volume as soon as it is expelled. In that way, spills and moisture are avoided providing also comfort to the user.
All around the world, disposable diapers, sanitary napkins, and compresses are used based on superabsorbent hydrogels. It is therefore, that developing these products and other similar products with biodegradable or reusable characteristics is one of the main goals of modern industry.
 Diaper absorbing enough liquid.
Diaper absorbing enough liquid.
Image credits
Toys.
Everyone has ever seen those toys of animals or superheroes that when immersed in water, grow. Yes, they are made of polymeric materials, specifically hydrogels.

Image credits
Reservoir for plants and crops water supply..
In this particular application, it is necessary to emphasize the importance these crosslinked polymers are having worldwide. Currently, and due to the high human population, the technique of agriculture must feed almost 7 billion people. To carry it out, millions of tons of fertilizers and pesticides are required annually, because is necessary to increase the yield of the crops, since spatially speaking, the available lands are being insufficient and the protected areas should not be used for these purposes. Also, many soils are very poor in nutrients for the development of crops. In addition to this, a high percentage of fertilizers applied to soils is lost by volatilization or filtration without being used efficiently by the plants. Not enough, these compounds, rich in nitrogen and phosphorus, end up in bodies of water, exaggeratedly increasing the concentration of nutrients in these spaces, thus generating eutrophication (proliferation of algae) and contributing to global warming, in the case of escaping into atmosphere.
The function of hydrogels in soils is to absorb water from rain or irrigation, as well as fertilizers and release them gradually, so that plants can supply them without losses to the environment and thus satisfy the need for plant growth and the improvement of agricultural production. There are many ways to use these materials for this purpose, such as mixing them with the soil next to plants, seeds and the same fertilizers. In contrast to the hydrogels incorporated in diapers, these are optimized for their ability to release water, instead of retaining it; which is one of the strengths of these materials, ranging from gardening, through the release of drugs, up to genetic engineering.
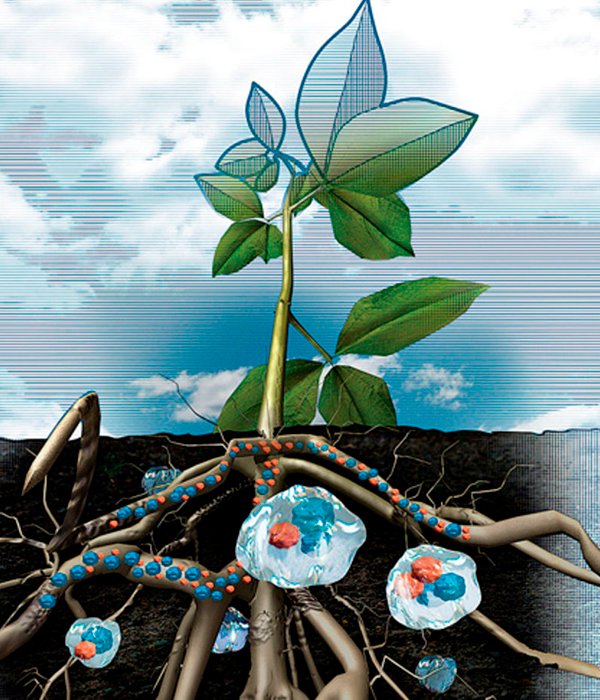
Plant absorbing water and nutrients through hydrogels.
Image credits
 Image credits
Image credits
Not only hydrogels are used to maintain the moisture of the cultivated soil and as absorbent materials (diapers and towels), but also to serve as coatings, microcapsules, membranes, catalyst support, etc. In addition, they mainly stand out in the biomedical area; which implies that these polymers meet a series of requirements, such as biocompatibility, which I have already mentioned. Also, that remain unchanged or not (depending on the goal) under degradative processes, have strength and mechanical properties that are suitable for a specific purpose. In short, they offer a biomimetic behavior, similar to that of biological systems. Below I summarize only some biomedical applications that I consider to be outstanding without diminishing the importance of others:
Applications in tissue engineering.
When some organs or parts of a set of tissues in the human body fail or suffer injury, there are several options for treatment. This may include repair, replacement or replacement by something synthetic or natural for a regeneration to occur; and this is where the tissue engineering comes in.
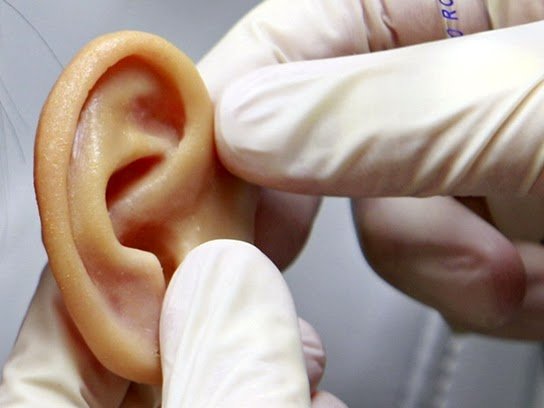
Currently, there are researchers working on the creation of prostheses such as ears ones, based on hydrogels.
Image credits
Hydrogels can act as three-dimensional polymeric scaffolds for these applications. A particular case is the so-called "tissue regeneration in vivo", where the affected patient's own cells are combined with the polymer, keeping in vitro until they can be implanted. The hydrogel will act as a natural extracellular matrix (remember that once swollen, hydrogels are composed of practically water, which gives them biocompatibility), a fact that will subsequently promote cell proliferation and consequently, tissue growth. These are promising studies for the future of medicine.
Hydrogels in contact lenses.
One of the reasons why hydrogels were synthesised, was for the realization of contact lenses. Soft contact lenses with thin thickness not only serve to correct problems of vision or aesthetic reasons, but also to maintain moisture in the eye. Remember that the eye organ should always be hydrated, these lenses to be in contact with the cornea absorb water from the eye environment and therefore maintain the humidity in that environment, they also offer oxygen permeation.
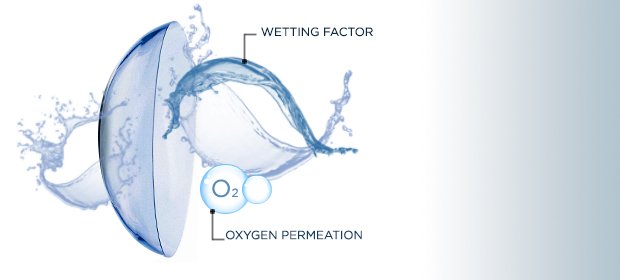 Image credits
Image credits
Applications in the controlled release of drugs.
With the methods that are usually carried out for the release of drugs for therapeutic purposes, poor controls are obtained on the optimal levels of the active ingredient demanded by the dosage, which causes that the medicine is not administered neither in the quantity nor in the time required for such treatments to be effective. Progress in hydrogels with drug releasing properties reveals a new alternative for a better control in the administration of medicines.
There is a large amount of officially admitted substances that are used as excipients for the different forms of drug transport. The functions of the excipients are very varied and of fundamental importance. Hydrogels are good candidates for excipients due to their high biocompatibility, water content, adjustable synthesis methods, three-dimensional physical structure, and ability to improve pharmacokinetics with longer circulation times allowing their functionalization directed specifically to the tissue or organ of interest.
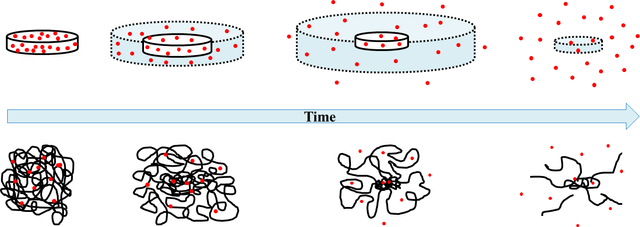 General scheme that shows how the active ingredient of a pill is released from a polymeric matrix.
General scheme that shows how the active ingredient of a pill is released from a polymeric matrix.
Image credits
They have also created a kind of patches to regenerate tissues damaged by burns. In those patches, the hydrogel due to its moisture helps to keep the area hydrated and free of bacteria, this when in in the polymer matrix a series of drugs can be incorporated. Example: patches with the antibiotic vancomycin.
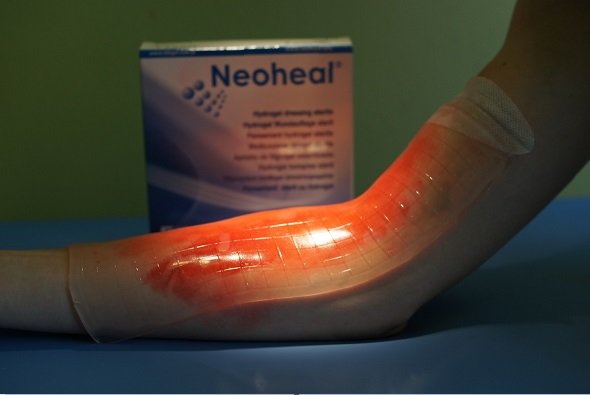 Image credits
Image credits
One of the research I have done with hydrogels.
As part of my research, a series of hydrogels based on acrylamide (AC) as main monomer and maleic anhydride (AM) as a comonomer, were synthesized. In order to provide biodegradability to the final cross-linked polymer, an interpenetrated polymer network of natural origin, specifically cassava starch, was incorporated into the matrix.
The hydrogel formulations were prepared by varying the proportion of AC/AM monomers, the amounts of cross-linking agent and starch. The synthesis was carried out in glass reactors (test tubes) by free radicals polymerization reaction in aqueous solution, during 2 hours at 75 ° C.
The obtained hydrogels were washed, dried and lyophilized for their characterization by infrared spectroscopy (FTIR) and scanning electron microscopy (SEM). The morphological studies by SEM showed the interpenetrating character of these polymers, and by FTIR the chemical environment of each formulation was checked. The study of the kinetics of absorption in distilled water showed that the hydrogels reached swelling percentages of up to 7500% in about 600 hours at 25 ° C, which classifies them as superabsorbents.
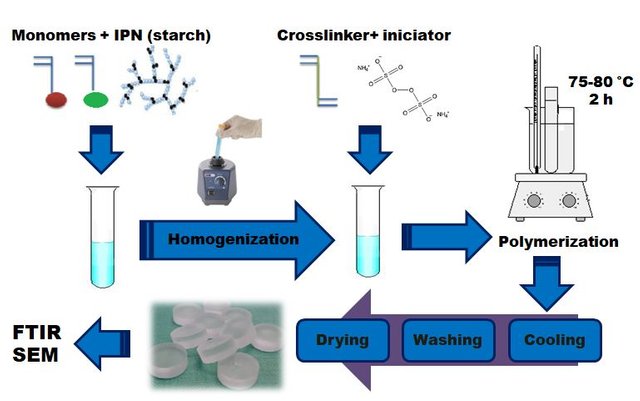
General scheme of the preparation of the hydrogels.(Imagen of my property)
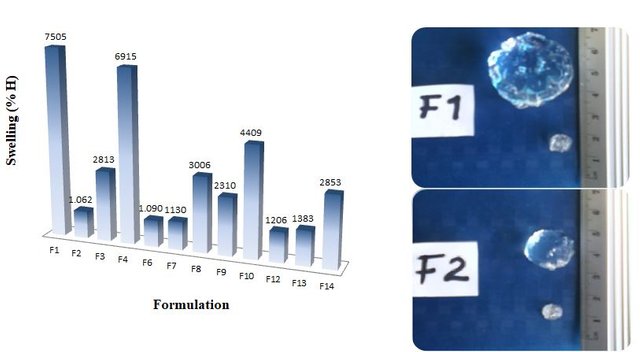
The high swelling percentages of the prepared hydrogel formulations can be observed, even the lowest exceeding 1000%. In addition it can been noticed how these materials grow after having been in contact with water.(Imagen of my property.)
The obtained hydrogels were evaluated in their absorption capacity of Na+, K+, Mg+2, Ca+2, Cl- and urea present in a synthetic simulating human urine solution. From that values of up to more than 80% absorption of the species in solution, were obtained, concluding that the synthesized hydrogels are potential components of absorbent materials for daily use.
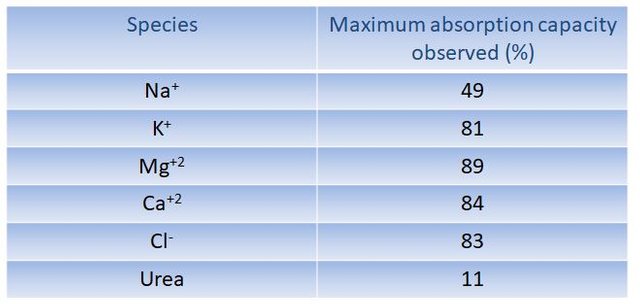
Absorption capacity of simulated urine elements, by prepared hydrogels.(Imagen propia.)
In future posts on
#steemstem community, I will discuss more investigations in this direction. In particular, I will address my last work in biodegradable hydrogels and their potential applications, for which this post precedes as an overview.
Conclusions.
As above demonstrated, hydrogels are promising materials. They have potentials applications in everyday life, in industry, in pharmacy and biomedicine mainly, in agriculture, and in environmental conservation. Hydrogels are also relatively easy to prepare from relatively economical monomers from which desirable chemical and mechanical properties can be controlled.
In regards the experimental part, interpenetrated hydrogels based on acrylamide and starch were synthesized. For that the free radical polymerization method in aqueous solution was used. It turned out that hydrogels reached a water absorption capacity of up to 7500% w/w, being superabsorbent hydrogels. The simulated urine absorption capacity in most of the elements present in the solution was higher than 80%. That feature certainly makes hydrogels potential candidates for manufacturing absorbent systems of daily use. With this potential, diapers, for instance, could be made much thinner and more comfortable to the current ones, reducing production costs and contributing to the conservation of the environment as they are biodegradable.
References:
(1) Lohani A; Singh G; Bhattacharya S; Verma A; Review Article “Interpenetrating Polymer Networks as Innovative Drug Delivery Systems” Publishing Corporation Journal of Drug Delivery, Volume 2014, ID 583612.
(2) Zhang Y; Chan HF; Leong KW; “Advanced materials and processing for drug delivery: The past and the future”, Advanced Drug Delivery Reviews. 2013, 65 (1): 104-120.
(3) Sartore L; Vox G; Schettini E; “Preparation and Performance of Novel Biodegradable
Polymeric Materials Based on Hydrolyzed Proteins for Agricultural Application”, Journal of Polymers and the Environment. 2013, 21 (3): 718-725.
(4) Laftah W; Hashim S; Ibrahim A; “Polymer Hydrogels: A review”, Polymer-Plastics Technology and Engineering, 2011, 50: 1475–1486.
(5) Odian G; “Principles of polimerización”, 4ta Ed, 2004, Jonh Wiley & Sons, Inc.
(6) Cano E; Urbina M; “Polímeros inteligentes y sus aplicaciones”, Informe de vigilancia tecnológica, Parque Científico Universidad Carlos III de Madrid, España, 2009.
(7) Soto D; Oliva H; “Métodos para preparar hidrogeles químicos y físicos basados en almidón: una revisión”, Rev. LatinAm. Metal. Mat.; 2012, 32: 154-175.
(8) Hoffman A; “Hydrogels for biomedical applications”, Advanced Drug Delivery Reviews, 2012, 64: 18–23.
(9) Sabino, M.; Escobar, O. "ESTUDIO DE HIDROGELES INTERPENETRADOS BIODEGRADABLES EN BASE A ACRILAMIDA PARA SU USO EN LA SORCIÓN DE BIOMOLÉCULAS”, Trabajo Especial de grado, Universidad Simón Bolívar, Coordinación de Maestría en Química, 2017.
Pictures can be found in the links. Schemes, work or pictures of my property are likewise indicated.
Thank you for reading my post! I am looking forward to hearing your comments, questions, criticisms or corrections, all of them are very welcome. See you next post.






















Hi @oscardice good luck with your research. Remember to be careful when working with acrylamide as it is a potent neurotoxin.
Cheers!
Ian
Thanks for reading and for your advice. Yes, in fact acrylamide has that risk, and we take safety measures in the laboratory. However, once it is polymerized, it loses that effect. For that reason, it is widely used to synthesize hydrogels, especially for agricultural purposes. Grettings from Venezuela.