Radiation Zoo: Ionizing radiation types, and how to detect/shield them.
Hello everyone. Since I really enjoy radiation-related topics, I wanted to write a guide of sorts of the different types of ionizing radiation, and how to protect against or detect them. I hope this post improves your understanding of the most common types of ionizing radiation.
If you already know the basics of ionizing radiation, you can skip the first section.
What is ionizing radiation?
It's important to distinguish between ionizing and non-ionizing radiation. Ionizing radiation can break molecular bonds and/or ionize atoms depending on its energy, while non-ionizing radiation cannot. The cutoff for "ionizing" usually occurs above around 10 electronvolts (eV) of energy per particle. For some perspective, the photons that make up the visible light that you can see have energies around 2 eV. 1 electronvolt is about 0.00000000000000000016 Joules, whereas your cell phone likely consumes 2 or 3 Joules every second to stay on.
The reason it's important to bring up this distinction is because many commonly talked about sources of radiation are NOT radiation in the commonly referred to sense. Microwaves are not nuclear radiation, do not ionize atoms, and cannot cause cancer. For example, the typical microwave oven produces electromagnetic waves with photon energies of 0.0000099 eV. If you wouldn't feel rich owning 0.00001 Bitcoin, you shouldn't feel worried about microwaves and radio waves giving you cancer. Obviously being inside a microwave oven would be harmful, but this is due to the overall energy being delivered (which will heat up objects), not the energy per particle (photon), which determines if particles can ionize atoms and cause damage to your DNA.
What kinds of ionizing radiation are out there?
This guide will cover the most common types of radiation, most of which you encounter in your every day life without realizing it.
Alpha Radiation
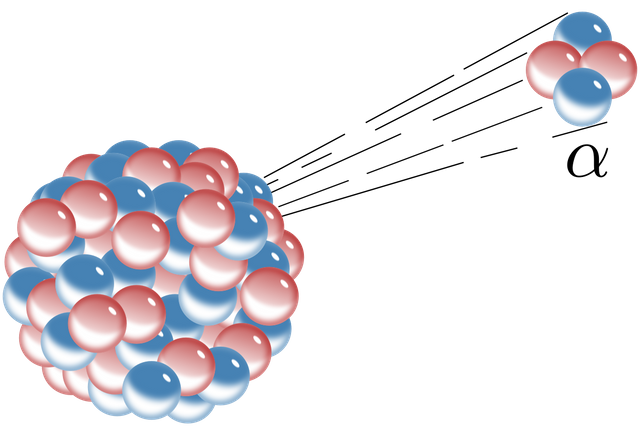
(Image credit: Wikipedia)
Many very heavy nuclei, including all Thorium and Uranium isotopes, are energetically unstable and will randomly (in accordance with their half-life) decay to a lower energy state. One of the more dramatic decays is alpha decay, which is very common in heavy unstable elements. In alpha decay, a Helium-4 (2 protons, 2 neutrons) nucleus breaks free of the parent nucleus and flies off with high kinetic energy (usually around 5 MeV, or 5 million electronvolts) while the original nucleus (now a different element, as it has lost 2 protons) recoils in the opposite direction. Because the original nucleus is now in an excited state, alpha decay almost always produces a secondary gamma photon (see below for discussion of gamma radiation).
While alpha radiation typically has very high energy as far as radioactive decay goes, it is actually one of the most harmless forms of ionizing radiation, provided it does not enter your body. Outside your body, alpha radiation is ridiculously easy to shield, and will not penetrate your skin or a piece of paper. Because of this, you can rest easy being in the presence of an alpha source if the gamma emission is not too high, PROVIDED YOU DO NOT CONSUME THE SOURCE. Once an alpha emitter source is inside your body (typically breathed in or eaten), alpha radiation becomes the most dangerous common type of radiation as there is no longer any skin to protect your inner cells.
That being said, it would be extremely difficult to eat enough alpha emitting material to cause significant harm. Common smoke alarms contain alpha emitters in harmless quantities. Alpha decay is also responsible for the majority of the Earth's internal heating.
Because alpha radiation is so easy to stop, most Geiger and photodiode detectors will NOT detect alphas. However, special chambers called "Ionization chambers" (which are very easy to make on your own, and is something I want to build in the next week or two) made by saturating the air inside of a container will alcohol easily detect alpha radiation by producing visible tracks in the air showing where the radiation passed through.
Beta Radiation
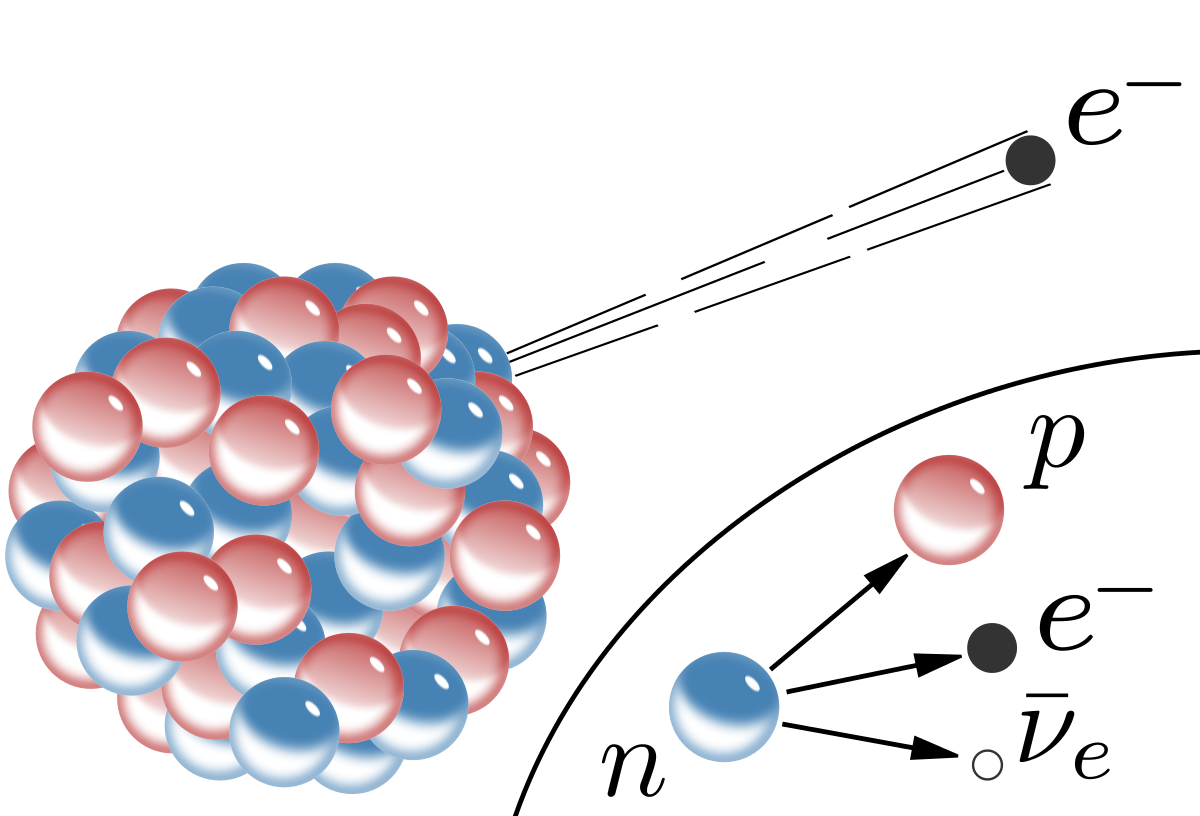
(Image source: Wikipedia)
Beta radiation, or electron radiation, is nothing more than high-energy electrons. Beta radiation is also a common output of radioactive decay. Beta radiation is commonly produced by heavy elements decaying via the weak force. In this decay, a proton emits an electron and electron anti-neutrino, converting itself into a neutron. Beta radiation is usually lower energy than alpha radiation when produced by decaying atoms, but it doesn't have to be. Because electrons have much lower mass than Helium nuclei (alphas) and a lower charge magnitude, beta radiation is much more difficult to shield than alpha radiation. That being said, it still isn't too difficult for common beta radiation energies, and for more most radioactive sources a sheet of aluminum foil will block essentially all beta emissions.
For very high beta radiation, another form of radiation comes into playing: Breaking radiation, or bremsstrahlung. When a charged particle accelerates or deccelerates, it produces electromagnetic waves (photons). If a beta particle strikes a nucleus head-on, the acceleration is so great that the secondary braking radiation produced is also ionizing, and is called an X-ray (X-ray machines use high-voltage to artificially accelerate electrons, which then strike a metal plate and produces both braking X-rays and characteristic X-rays produced when electrons are knocked out of their orbitals).
Because of braking radiation, the best way to shield against beta radiation is lots of low density material. Higher density material will better block the actual beta radiation, but will produce more secondary braking radiation when electrons hit the larger nuclei.
As a side note, there is a also "Beta-Plus" radiation, which is exactly the same as normal beta radiation except the particle involved is a positron. Positrons are the antimatter equivalent to electrons, so after they are stopped in a material, they will annihilate with an nearby electron and produce gamma radiation.
The anti-neutrino I mentioned earlier is also technically ionizing radiation, but can only interact via the weak force and gravity. As such, neutrinos are pretty much the most harmless particle in the universe, and will never cause any damage whatsoever to anything.
Almost all geiger counters and photodiode detectors will detect beta radiation, both of which are somewhat easy to make at home as a DIY project.
Gamma Radiation
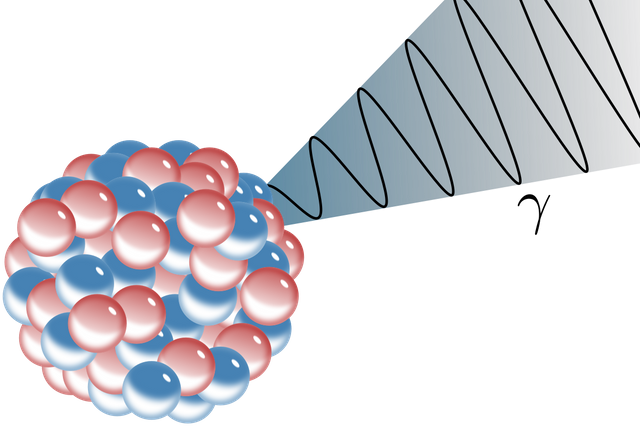
(Image credit: Wikipedia)
Gamma radiation is just very high energy photons. They are identical to X-rays but typically have higher energy (X-rays are photons produced by electrons (energy level transitions or braking radiation), while gamma rays are photons produced by nuclei. The distinction is not really important).
Gamma radiation typically isn't absorbed by materials (or people) as easily as the other two types I've already discussed, but they also generally are much harder to stop/shield. Because of this property, they are picked up by essentially any radiation detector, although ionization chambers will have a harder time detecting them since they are uncharged and the chamber will only pick up secondary events.
Since gamma radiation is made of electromagnetic waves, the best shielding material the densest material you can find, so that the shielding material has many electrons available to absorb or scatter the gamma rays.
Gamma radiation is produced by various radioactive decays. In electron-capture decay, the nucleus absorbs one of the atom's electrons, turning a proton into a neutron and releasing a gamma ray. The byproducts of nuclear fusion, fission, and alpha-decay can also often produces gamma rays as they drop down to their ground energy states. How effective shielding will be really depends on the energy of the gamma photons, which can vary wildly depending on the process that produced them. In general, materials like lead will work well, or lots and lots of lower density material.
Muon Radiation
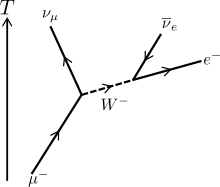
(Image credit: Wikipedia)
Muon radiation is unlike the previous radiation types mentioned in that it is never produced in radioactive decay. Muons are identical to electrons except their mass is over 400 times the mass of the electrons. Because of this mass difference, they decay very quickly (typically a few microseconds) into neutrinos and an electron.
Muons are produced high in the upper atmosphere when cosmic radiation (extremely high energy protons, electrons, and nuclei from outside the solar system) impact atmopsheric air, producing pions, which then decay into muons. Muons are able to reach the ground before decaying due to relativistic time dilation, and are extremely difficult to shield without going underground. As natural background radiation, they aren't dangerous, and there's nothing you can do to stop them. Muon radiation is partially the cause of background radiation clicks on Geiger counters.
Since muons get through almost any reasonably sized shielding, they can be used to image the inside of sealed objects, and have even been used to scan the insides of the pyramids.
Other types
Unfortunately, this guide would never be able to cover all of the types of ionizing radiation. In addition to cosmic radiation (including protons, electrons, positrons, and heavy nuclei, all moving at ridiculously high speeds and energies), there is solar wind (low energy nuclei and electrons, but still ionizing), Van Allen belt proton radiation, pions and other unstable particles, and neutron radiation (which I may have to talk about later, as these are still pretty relevant). Most of the above (with the exception of neutrons) are very rare on Earth and not very relevant.
Unless you live next to a reactor meltdown, none of this will change your life. But, I hope that you were able to learn something new from this post!
If there is any interest, I would like to write a guide on different types of radiation detectors that anyone can build in their garage for relatively little money in the future.
If you enjoyed this post or learned something from it, and would like to see more in the future, be sure to upvote. If you have any questions, ask away in the comments and I'll do my best to answer.
Thanks for reading!
Seems like you put a lot of effort in this post, well done!
I'd love to see some references and image credits in active links ;)
In my opinion, the presented factual information in this post qualifies as common knowledge. The shared information can be found in a large number of sources and can be considered as non-controversial. Therefore, no obligation to add references. Having said that, to decide whether a given piece of information is common knowledge or not can be a matter of perspective.
Perspective exactly! For someone new into this further studying is needed. That's why we are for active links, so that anyone interested can check out for themselves ;)
Those topics are important because the majority of the general public doesn't get the sense about the radiation effect, especially for alpha and beta. Follow and vote
Thank you for that, sweet, sweet information. Keep it coming!
Science is weird.. The more we get into the atom, we find bigger things.