Characteristics of The Nucleus, Natural Radioactivity and Table of nuclides
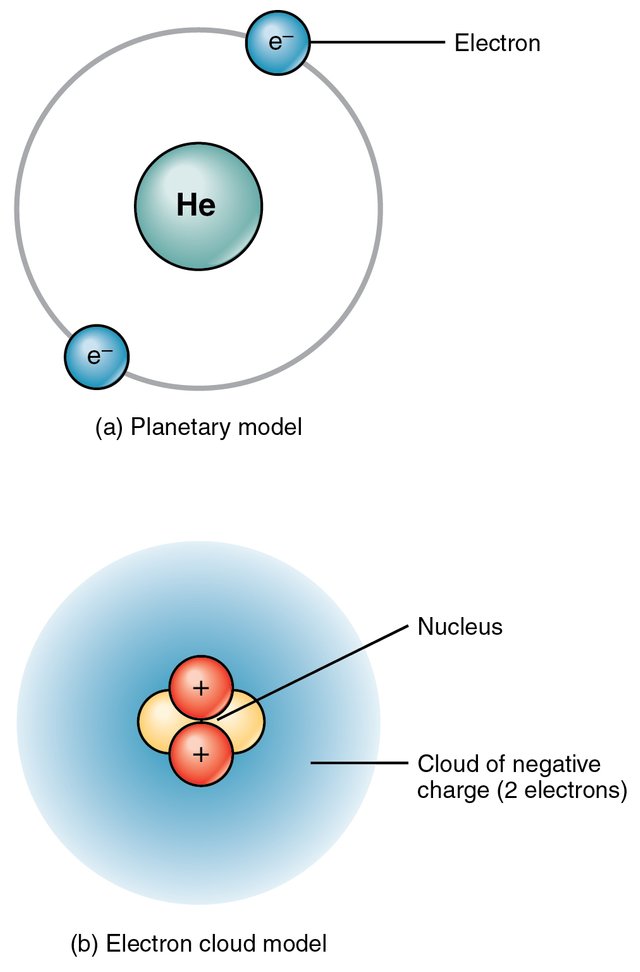
Most of you who have a bit of science background knows that the atom is made of nucleus and electrons orbiting around it. The nucleus is positively charged and the electrons are negatively charged. In total, the atom is electrically neutral. As I am going to write about nuclear physics, I will talk about mostly about the nucleus and the phenomenons are happening in it. Believe it or not, although the nucleus is so small it has almost all the masses of the atom and it can unleash such an amount of energy that we can light a whole country.
So, what is inside the nucleus? Well, all of you probably know that it is made of protons and neutrons. The proton number defines which elements it is in the periodic table and the sum of the proton and neutron number gives the atomic mass of the atom. But it can happen that two or three have the same atomic number but a different mass number. They are called the isotopes. They fall in the same element in the periodic table just a bit heavier. Sometimes the mass number is the same but they have a different number of protons and neutrons. They are called the isobars Another thing is when the neutron number are the same they are called isotones.
Radioactive series
Most of the naturally radioactive elements don't exist anymore as their half-life is less than the age of the earth. But some of them are still there. We divide them into four. There can be a mathematical calculation behind it. Like we can divide the mass number(A) by 4 we get the Thorium series. if you divide by 4 and then add 1 you get the Neptunium series, this way Uranium and Actinium series. Radioactivity is one of the most interesting topics in nuclear physics. It will be interesting to you perhaps when I will start writing about the fission and fusion reactions. As I wrote about alfa and beta decays. I will write here in short but I will write individual posts for them as well. So, alfa decay is actually a quantum mechanic tunnel effect. It tunnels through the nucleus. In Alfa decays the nucleus loses 2 protons and 2 neutrons and completely change into another atom. In beta decay, the mass number doesn't change. It can happen in three ways- Beta plus decay, beta minus decay and electron capture. In beta plus one proton from the nucleus convert to a neutron and it can on happen in the nucleus as it needs threshold energy and beta minus covert one neutron to one proton and in electron capture the nucleus capture an electron from the nearest shell.Table of nuclide
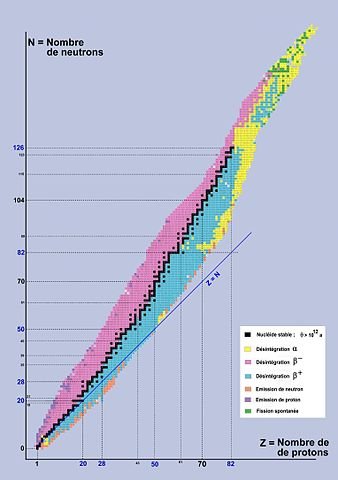
The important thing is nuclear stability. So, when in the nucleus the proton and the neutron numbers are the same it is more stable. But as the proton number increases the neutron number has to increase more than the proton to compensate it so that the nucleus doesn't break apart. So, if we make a graphical representation between the neutron( in y-axis) and proton (in x-axis) we will find a linear shaped graph until the proton and neutrons are the same. Then it takes a shape of a curve. Bellow the line happens beta plus and electron decay as it wants to decrease the amount of neutron and come to stability. Above the line, its beta minus, just to increase the neutron numbers to be more stable. Then near the curve happens alpha decay and in the end, those heave nucleus decays to come to stability. I will talk next time about nuclear fission and how can we get electric energy from nuclear fusion.
So, that will be all for today. Hope everyone will have a nice week.References
1. Povh, Nuclei and particles
2. Jean-Louis Basdevant, James Rich, Michel Spiro, Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology
3. Burcham, Nuclear physics, an introduction
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @curie.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
This post was shared in the Curation Collective Discord community for curators, and upvoted and resteemed by the @c-squared community account after manual review.
@c-squared runs a community witness. Please consider using one of your witness votes on us here