The ‘holy’ water
Okay! Without creating any sort of drama or something let me tell you a secret: your water has been hiding something from you. Your water has a secret! So, suddenly now you think it’s has been unfaithful to you. Let your prejudice on and without once questioning about the secret, judge the bloody water! Go on, this is what we’re accustomed to!
Or there is a second option, which you might not realize but always is, has and will be. Those of you science enthusiasts might just have guessed about the thing that I am going to talk about but for those of you who aren’t much into science but are interested to know about the crazy property or “secret”, without further due, let’s!
The normal or mainstream thing is when you heat up an object it expands and when you cool it, it shrinks. And this is just about right for every goddamn metals, nonmetals. But water, water is different. It follows the trend on heating and also cooling but there’s a catch. It behaves like every other object on cooling but if you cool past 4°C, it expands. This is why water has the highest density at 4°C. What is density? Well, density is the amount of mass over a unit volume and has a unit of kg/m3 in SI.
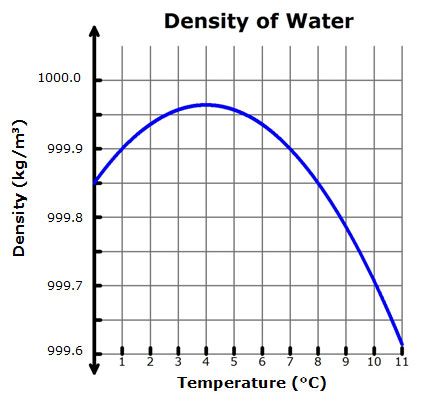
Technically, this property of water is termed as anomalous expansion. you might not feel this but this thing I just told you about answered a lot of phenomenon happening round us. Let’s see some examples! This property is why ice floats on water because density of ice at 0°C is 916.8 kg/m3 whereas that of water is 999.8 kg/m3. (Titanic? Ah, that’s why). And this property is why pipes outburst in winter. Why? Because the volume of ice is greater than that of water flowing and hence the low density.
This property also answers your skating problem. Why is this whole stupid lake not ice? Why there has to be water beneath this layer, why didn’t the entire lake or pond freeze up? Heavier objects sink while lighter objects float. Ice, formed when the temperature of surrounding reaches or falls below 0°C, having the lowest density floats on the upper surface or forms the upper surface of the frozen say pond, while layer having density higher than that of ice tend to sink down. As we know, these difference in densities are related to temperature and hence we’ve a temperature gradient as we go down the pond and this is why aquatic organisms stay alive in winter. Nature works is a mysterious way!
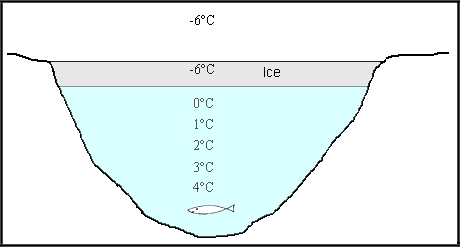
So, is it you or the water? You decide.
P.S. For those of you wandering about the cause of this property, I have included a link down below in the reference section. But in short, hydrogen bond occurring in the water molecule is responsible for this phenomenon.
REFERENCES