Basic concepts about carbohydrates and lipids: important factors of our daily life
In recent years, there has been great progress in understanding how carbohydrates and lipids influence nutrition and human health. The progress in scientific research has highlighted the various functions that these have in the body and its importance to enjoy good health. Below we will see different concepts to understand more about this topic.
Carbohydrates
Carbohydrates are biomolecules made up of carbon, hydrogen and oxygen atoms, in the proportion indicated by their empirical formula. They may exceptionally contain atoms of other elements, such as nitrogen, sulfur or phosphorus. These compounds account for up to 90% of organic biomolecules in some organisms; hence its biological importance.
They are also known as carbohydrates or carbohydrates, because, initially, it was thought that they were formed by a carbonated structure hydrated with water molecules; today it is known that this is not the case, although its empirical formula may suggest it.

Source
Chemically, carbohydrates are aldehydes and ketones with multiple hydroxyl groups. The more complex ones contain, in addition, other organic functional groups.
The simplest carbohydrates are called osas or monosaccharides. The union of these monomers gives rise to more complex molecules called osidos, that can contain a variable number of bears and even associate to other different molecules, like lipids and proteins.
- The osidos
The ósidos can be classified in several groups:
• Holosidos: They are ossidos constituted only by bears. According to the number of monomers bound, they differ:
• Oligosaccharides: Contain between 2 and 10 monosaccharides. The most important are disaccharides, which result from the union of two monosaccharides.
• Polysaccharides: They are formed by multiple repetitive units of monosaccharides. By their composition, they are divided into two groups:
• Homopolysaccharides. They are formed by the repetition of a single monomer.
• Heteropolisaccharides. Its composition is more varied, since they contain more than one type of monomer.
- Heterosides
They are complex compounds that arise from the combination of a set of monosaccharides with molecular fractions of non-glucidic nature, such as proteins, lipids or other diverse organic molecules: for example, alcohols and phenols.
- Classification of Carbohydrates
• Monosaccharides
The simplest carbohydrates are the Monosaccharides, which have as a general formula (cH2O) n. The value of "n" can vary from 3 to 7 and according to this value, monosaccharides are called triose, tetrosas, pentoses, hexoses and heptoses, respectively. Examples of monosaccharide are glucose, fructose, galactose, ribose and deoxyribose.
• Disaccharides
Disaccharides are molecules formed by the union of two monosaccharides. The formation reaction of a disaccharide is a synthesis by dehydration: one of the monosaccharides loses a hydrogen (-H) and the other loses an hydroxyl (-OH), the two monosaccharides are bound, and the hydrogen and hydroxyl released form a water molecule.
Sucrose (sugar cane sugar) is a disaccharide formed by the union of a molecule of glucose and one of fructose. Another example of disaccharide is lactose (milk sugar), constituted by a glucose linked to a galactose.
• Polysaccharides
Polysaccharides are large molecules, made up of hundreds or thousands of monosaccharides. Some examples of polysaccharides are starch, glycogen, cellulose, chitin, among others.
The glucose molecules manufactured in photosynthesis and bound in a certain way are transformed into starch, which is stored in the cells of plants, when the cell needs energy, the starch is broken by hydrolysis, transforming back into glucose molecules. Starch is the main storage substance of plants and many algae. Polysaccharide storage also occurs in our organism.
After feeding, the liver cells absorb glucose molecules from the blood, binding them together so that they form polysaccharides. When the volume of glucose in the blood decreases in the periods between the feeding, the cells of the liver break the glycogen converting it into glucose they are thrown into the blood.
The cellulose, substance that constitutes the wall of the plant cell and also a polysaccharide formed from glucose molecules. It is estimated that algae and land plants produce 10 million tons of cellulose daily.
Unlike starch and glycogen, cellulose is very resistant to digestion, only a few species of fungi, bacteria and protozoa are able to digest it. These microorganisms produce cellulose.
- The importance of carbohydrates for living beings
The energy necessary to form all the organic matter existing in the earth comes from outer space, more precisely from the sun. The light energy of the sun is captured by algae and plants, which use it to make glucose molecules. This sugar is preserved in the form of chemical energy. A good part of the energy was used in its manufacture.

Source
- Starch, glycogen and cellulose
• Starch: Starch is the reserve polysaccharide of plant cells, very abundant in the tubers and in some seeds, such as corn. It consists of two types of chains, the two formed by the union of alfadiglucosa: amylose, which is a long chain without branching, and amylopectin, which is a very branched chain.
• Glycogen: It is the storage form of glucose in animals. It is constituted by branched chains of alfadiglucosa. It is stored in the liver and skeletal muscle.
• Cellulose: It is a structural polysaccharide that forms the walls of plant cells. It is formed by linear chains of alfadiglucosa.
Lipids
Lipids are a group of biological compounds that are classified together by their structure, generally apolar (carbon, hydrogen and oxygen), which makes them poorly soluble in water. They consist mainly of fatty acids and glycerin or other alcohols. They are usually classified into glycerides (oils and fats), phospholipids, sphingolipids, glycolipids, céridos (waxes), steroids and terpenes. Fats and oils are the most abundant, these are the main constituents of the storage cells of these in animals and plants, and make up one of the important food reserves of the body. The difference between fats and oils is very clear; the oil is a liquid at room temperature, while the fat is solid. These can be extracted from animals and vegetables, thus obtaining substances such as corn oil, coconut oil, palm oil, tallow, bacon fat and butter.
Source
From the chemical point of view, they are esters of fatty acids, formed by esterification reactions between these and an alcohol (glycerol), to each molecule of glycerol three fatty acids are united, from which the word triglycerides is derived. Fatty acids are made up of long hydrocarbon chains, saturated (with simple bonds) or unsaturated (with double bonds). Animal fats tend to be saturated, while most oils are unsaturated (except palm oil, coconut and cocoa butter).
- Fatty acids
The fatty acids are long chain monocarboxylic acids. In general, they contain an even number of carbon atoms, usually between 12 and 24. This is because their biological synthesis takes place through the successive apposition of units of two carbon atoms. However, there are also fatty acids with an odd number of carbon atoms, which probably derive from the methylation of an even chain fatty acid.
The chemical properties of the fatty acids derive, on the one hand, from the presence of a carboxyl group, and on the other hand from the existence of a hydrocarbon chain. The coexistence of both components in the same molecule converts the fatty acids into weakly amphipathic molecules (the COOH group is hydrophilic and the hydrocarbon chain is hydrophobic). The amphipathic character is greater the smaller the length of the hydrocarbon chain. The solubility in water decreases as the length of the chain increases.
- Lipid functions

Source
Reservation: they constitute the main energy reserve of the organism. It is known that one gram of fat produces 9.4 Kc. In metabolic reactions of oxidation, while protids and carbohydrates only produce 4.1 Kc./gr. Oxidation of fatty acids in the mitochondria produces a large amount of energy. The fatty acids and fats (Acylglycerides) constitute the main reserve function.
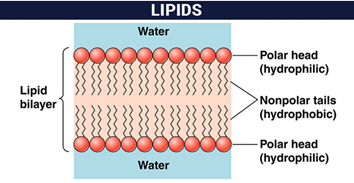
Structural: They form the lipid bilayers of cytoplasmic membranes and cellular organelles. Phospholipids, cholesterol, Glucolipids etc. they are in charge of fulfilling this function.
In the organs they cover structures and give them consistency, such as hair wax. Others have thermal function, such as acylglycerides, which are stored in the adipose tissues of cold weather animals.
They also protect mechanically, as in the adipose tissues of the sole of the foot and in the palm of the man's hand. In summary: the structural function is entrusted to Glucolipids, Céridos, Sterols, Acilglicéridos and Phospholipids.
- Transport. The transport of lipids, from the intestine to the place of use or to the adipose tissue (storage), is carried out by the emulsion of the lipids by bile acids and proteolipids, associations of specific proteins with triacylglycerides, cholesterol, phospholipids, etc. , which allow their transport by blood and lymph.
- Phospholipids
Phospholipids in general are those lipids that contain phosphoric acid. In the field of food science and technology, expression is usually limited to glycerophosphoric acid derivatives, which are formed by a glycerol molecule esterified at positions 1 and 2 by two fatty acids, with position 3 esterified by a phosphoric acid that also carries other structures, depending on the phospholipid in question. Generically they are called "lecithins", although it is considered that lecithin itself is phosphatidylcholine.
According to the structure linked to phosphoric acid, we can talk about phosphatidylethanolamine, phosphatidylcholine, phosphatidylserine, and phosphatidylinositol, which are the most frequent phospholipids in food.
The structure of the bound fatty acids also varies among the phospholipids. In phosphatidylcholine it is usual for the fatty acid that occupies position 2 to be unsaturated, while the one that occupies position 1 to be saturated, as shown in the figure. In phosphatidylethanolamine the opposite often happens. Phospholipids are polar lipids, since they have a hydrophilic region formed by the charged phosphate group and the hydrophobic zone of ethanolamine, choline, serine or inositol.
Phospholipids are the main lipid constituents of biological membranes, where they form bilayer structures, with the non-polar zones of the constituents of each layer oriented inwards. Consequently, phospholipids will be present in most complex foods, in which cellular material exists. Phospholipids are also capable of producing artificial structures of the bilayer type (liposomes).
Their interest in the field of food is that they are polar lipids are oriented on the contact surfaces between water and hydrophobic materials, and reduce surface tension. That is to say, they reduce the energy necessary to create a contact surface and the tendency to minimize it, facilitating the production of emulsions and stabilizing them.
Phospholipids are an important component of egg yolk lipids, which explains their good capacity as an emulsifier. It is also found in the membrane of the fatty globule of milk (and consequently, in butter). The phospholipids used as emulsifiers in industry (lecithins) usually come from the refining of soybean oil.
Thanks for reading me.
For more information visit the following links: