The origin of life, organization levels and biochemistry
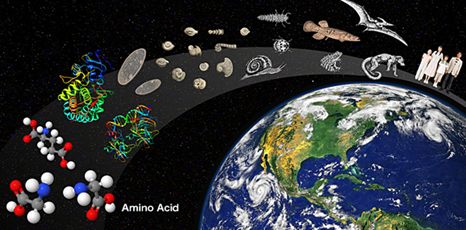
One of the most fascinating topics in the natural sciences is found in the theme of life, how and when did life originate? Life is the result of a spontaneous generation of inert life that was opened millions of years ago. step for certain molecules to be duplicated giving rise to processes that today we call life, or was the life planted or by a superior being (religious theory) or came from stones or other objects from space and that somehow are " seeds "found the favorable ground to duplicate themselves and generate life (theory of panspermia). As you can see we find a whole branch of science in biology that tries to explain us about how life originated, and in our interior it is also a question of diligently and frequently comes to our mind and somehow find an answer defines us in many fields, as are our beliefs and principles. For our case we will take a look at the theories of life from the biology that is the field of action of this blog.
Source
- The atmosphere of the primitive earth
It is commonly admitted that the primitive atmosphere, about 4400 million years ago, was very thin and reductive, as it lacked almost completely oxygen. According to Oparín, it would have been formed by compounds such as ammonia and carbon dioxide.
The temperature was very high, the electrical storms followed one another incessantly and the radiations of the sun penetrated with ease to the surface of the planet. The activity of the volcanoes was high and they began to expel into the atmosphere in the form of vapor from the concentrated water in the interior of the earth. The steam condensed forming the first oceans about 4200 million years ago. The atmospheric conditions had to allow the combination of certain molecules, so that they were repeated millions of times, getting to be combined with other different ones.

Source
It is believed that the first living thing appeared about 3900 million years ago and, without a doubt, it was a simple organism, similar to a bacterium. About 3500 million years ago, some bacteria began to use sunlight in a process called photosynthesis, so they started to release oxygen in the form of microbubbles. The action of the oxygen emitted by the first photosynthetic beings-the cyanobacteria-would eventually alter the conditions for life on Earth.
- The first cell
It appeared 3,500 million years ago. 1,000 million years after the earth originated as a rocky body with atmosphere and ocean. It could be thought that the first cells were like the smallest and simplest organisms that live today, the microbes known as mycoplasmas.
The cells of mycoplasmas are really tiny, more than a billion times smaller than a protozoan, and harbor only a fraction of the DNA and proteins normally present in a cell. From a biochemical perspective, three characteristics distinguish living cells of other chemical systems:
- The ability to duplicate generation after generation
- The presence of enzymes, the complex proteins that is essential for the chemical reactions on which life depends
- A membrane that separates the cell from the surrounding environment and allows it to maintain a different chemical identity.
It is not known when the first living cells appeared on Earth, but we can establish a certain time scale. The earliest fossils found to date that resemble the current bacteria, date from between 3400 and 3600 million years, about 1000 million years after the formation of the Earth. Although the fossils are so small that their structure can only be observed with the electron microscope, they are complex enough to make it clear that some small aggregate of chemical substances would have transposed the zone of gloom that separated the living from the non-living, millions of years ago.
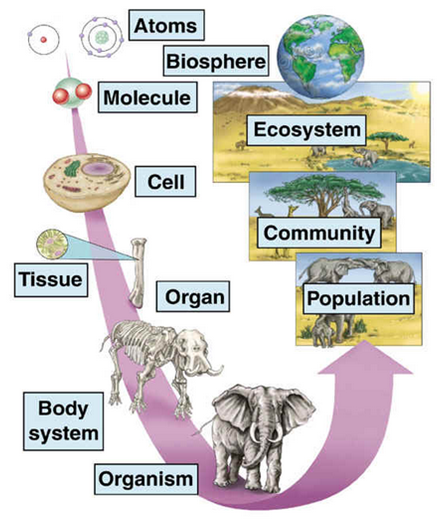
Organization levels
We know how Organizational Levels to the different degrees of complexity in which we can find organized the matter. That is to say, that in each of the levels there are elements that, joined together, form a more complex structure with different characteristics and new properties. In turn, this structure, by grouping with others like it, is capable of forming an even more complex matter.
For example, cells are made up of simpler elements. Later, the grouping of cells forms, among other structures, tissues and organs.
Source
Let's see now the classification of the different levels of organization and what we find in them:
1.) Molecular level: It is the abiotic level or non-living matter. At this molecular level there are four sub-levels:
• Subatomic sub-level: Subatomic particles constitute it; that is, protons, electrons and neutrons.
• Atomic sub-level: Constituted by atoms, which are the smallest parts of a chemical element that can intervene in a reaction
• Molecular sub-level: constituted by molecules; that is, by material units formed by the grouping of two or more atoms by chemical bonds (examples: O 2, H 2 O), and that they are the minimum amount of a substance that keeps its chemical properties we distinguish two types of molecules: inorganic and organic.
• Macromolecular sub-level: It consists of polymers that are the result of the union of several molecules (examples: proteins, nucleic acids). The binding of several macromolecules gives rise to macromolecular associations (examples: glycoproteins, chromatin). Finally, molecular associations can join and form cellular organelles or organelles (examples: mitochondria and chloroplasts).
Molecular associations constitute the limit between the biotic world (of living beings) and the abiotic world (of non-living or inert matter). For example, nucleic acids possess the capacity of author replication, a characteristic of living beings.
2.) Cell level: Includes the cell, anatomical and functional unit of living beings. The smallest structural unit of living beings capable of functioning independently. Each cell has a chemical support for inheritance (DNA), a chemical system for acquiring energy etc. There are two types of cells:
• Prokaryotic cells: those that lack nuclear envelope and, therefore, genetic information is dispersed in the cytoplasm, although condensed in a region called nucleotide.
• Eukaryotic cells are those that have the genetic information surrounded by a nuclear envelope, which isolates and protects it, and which constitutes the nucleus. Cells are the smallest parts of living matter that can exist free in the medium. Organisms composed of a single cell are called unicellular organisms, and must perform all vital functions.
3.) Pluricellular or organic level: Includes all living beings made up of more than one cell. In multicellular beings there is a division of work and a cellular differentiation reaching different degrees of increasing complexity:
• Fabrics: is a set of very similar cells that perform the same function and have the same origin. For example heart muscle tissue.
• Organs: Group of cells or tissues that perform a certain function. For example, the heart is an organ that pumps blood into the circulatory system.
• Systems: is a set of several similar organs that work independently and are organized to perform a certain function; for example, the circulatory system.
• Apparatus: Set of organs that can be very different from each other, but whose acts are coordinated to constitute a function.
4.) Population level: Living beings generally do not live in isolation, but are related to each other. A population is a group of individuals of the same species, living in the same area at a decisive moment and influencing each other. Groups of similar individuals that tend to mate with each other in a limited geographical area. This can be as simple as a field with flowers separated from another field by a hill without flowers, or a herd of goats in a field.
A Community is the relationship between groups of different species. For example, desert communities may consist of rabbits, coyotes, snakes, mice, birds and plants such as cacti. The structure of a community can be altered by things such as fire, human activity and overpopulation.
5.) Ecosystem level: The different populations that live in the same area at a given time form a community or biocenosis. The physicochemical conditions and the characteristics of the environment in which they live constitute the biotope. To the set formed by the biocenosis, the biotope and the relations established between both is called ecosystem.
6.) Biosphere: The sum of all living beings taken together with their environment. In essence, the place where life occurs, from the heights of our atmosphere to the bottom of the oceans or up to the first meters of the surface of the soil (or let's say better kilometers if we consider the bacteria that can be found to a depth of about four kilometers from the surface). We divide the Earth into atmosphere (air), lithosphere (firm earth), hydrosphere (water), and biosphere (life).
Biochemistry
Biochemistry is the science that studies the chemical components of living beings, especially proteins, carbohydrates, lipids and nucleic acids, as well as other small molecules present in cells. Biochemistry is based on the concept that all living things contain carbon and in general biological molecules are composed mainly of carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur. It is science that studies the very basis of life: the molecules that make up cells and tissues, which catalyze the chemical reactions of digestion, photosynthesis and immunity, among others.
- The bioelements
They are called biogenic elements or bioelements to those chemical elements that are part of living beings. Considering their abundance (not importance) they can be grouped into three categories:

Source
- Primary or main bioelements: C, H, O, N. they are the major elements of living matter, they constitute 95% of the total mass. The physical-chemical properties that make them suitable.
- Secondary bioelements: S, P, Mg, Ca, Na, K, Cl. We find them forming part of all living beings, and in a proportion of 4.5%.
- Trace elements: they are referred to as the set of chemical elements that are present in the organisms in vestigial form, but which are essential for the harmonious development of the organism.
The bio elements are important for the life of all living beings, the living matter is constituted by some 70 stable elements that there are in the Earth, except the noble gases. That is why we must protect the biodiversity of our planet's natural resources and make the most of them in a positive way; so for example using natural substances in different applications such as: solar energy, water currents, air, oxygen, nitrogen, and biomass, etc., to produce electrical energy, as energy from various technologies, or well as sources of energy for our agricultural soils, in short there are many applications; this in order to preserve organic life on the planet and a better quality for all living beings.
- Mineral salts
Mineral salts are inorganic biomolecules that appear in living beings in a precipitated form, dissolved in the form of ions or associated with other molecules.
Precipitated: The salts are formed by the union of an acid with a base, releasing water. In hasty form they form hard structures, which provide structure or protection to the being that owns them. Examples are shells, shells or skeletons.
Dissolved: Salts dissolved in water show positive or negative charges. The most abundant cations in the composition of living beings are Na +, K +, Ca2 +, Mg2 +. The most representative anions in the composition of living beings are Cl-, PO43-, CO32-.The salts dissolved in water can perform such functions as:
• Maintain the grade of salinity.
• Diminish pH changes, through the buffer effect.
• Control muscle contraction
• Produce electrochemical gradients
• Stabilize colloidal dispersions.
- Water, source of life
The importance of water in life can be understood if we refer to the functions performed by organisms to stay alive. In the functions that allow organisms to manage energy to synthesize and degrade compounds, water plays a decisive role. Likewise, organic compounds, energy source, are transported through water.
Photosynthesis could not take place in photosynthetic vegetables, without the presence of the water molecule. The light phase requires the breakdown of the water molecule (photolysis) to provide the electrons needed for the process. All organisms depend on the functions performed by plants (autotrophs) so that without water, this important link in the life chain, life as we know it would not be possible. Thus, water is at the same time an input and a vehicle. The circulation of both nutrients and waste uses water in organisms as a basic component of vital fluids.
Waste products from organisms also use water as a vehicle. We could say that any metabolic activity is intimately linked to the water molecule. On the other hand, the organisms establish intimate and transcendental relations with the environment. Water, thanks to its heat capacity, plays a very important role in the thermal regulation of the climate, making the variations less abrupt, than they would be if there was no water. In the body, water also has this important function: to regulate the temperature. The release of water vapor as sweat or panting is vital for the preservation of body temperature.
Organisms have structures that allow them to "capture" information about the environment that surrounds them. Sensory organs could not pick up olfactory and gustatory signals if the molecules they perceive were not transported by water. The reproductive functions and their transport are also closely linked to water.
- Organic molecules
In organisms there are four different types of organic molecules in large quantities: carbohydrates, lipids, proteins and nucleotides. All these molecules contain carbon, hydrogen and oxygen. They also contain nitrogen and phosphorus some lipids and nucleotide and nitrogen and sulfur proteins.
Carbon is uniquely suited to this central role, due to the fact that it is the lightest atom capable of forming multiple covalent bonds. As a result of this capacity, carbon can be combined with other carbon atoms and with different atoms to form a large variety of strong and stable chains and ring-shaped compounds. Organic molecules derive their three-dimensional configurations primarily from their carbon skeletons. However, many of its specific properties depend on functional groups. A general characteristic of all organic compounds is that they release energy when they are oxidized. Among the major types of organic molecules important in living systems are carbohydrates, lipids, proteins and nucleotides.
Source
Carbohydrates are the primary source of chemical energy for living systems. The simplest are the monosaccharides, as the main glucose source of choice of most heterotrophs, or sugar ribose of nucleotides. The monosaccharides can be combined to form disaccharides such as lactose component of milk or maltase component of the starch which is a polysaccharide.
Lipids are hydrophobic molecules that, like carbohydrates, store energy and are important structural components. They include fats and oils, phospholipids, glycolipids, waxes, and cholesterol and other steroids.
Proteins are very large molecules composed of long chains of amino acids, known as polypeptide chains. From only twenty different amino acids used to make proteins, a vast variety of different types of protein molecules can be synthesized, each of which fulfills a highly specific function in living systems.
Nucleotides are complex molecules formed by a phosphate group, a sugar of five carbons and a nitrogenous base. They are the structural blocks of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), which transmit and translate genetic information. Nucleotides also play central roles in the energy exchanges that accompany chemical reactions within living systems. The main carrier of energy in most chemical reactions that occur within cells is a nucleotide carrying three phosphates, ATP.
Thanks for reading me.
For more information visit the following links:
welcome to steemit world,
Hope you enjoy here
#Happy #steemiting!!!
btw i wrote about Crypto if you are interested in it you can check my Blog
@jibi333 [Crypto Boy]