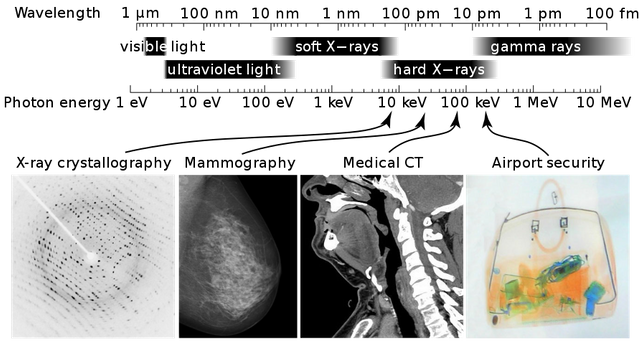
The production nature, properties and uses of X-rays
X-rays (Electromagnetic radiation in a wavelength range) were first discovered by a German physicist Wilhelm Roentgen in 1895. He found out that when a cathode ray tube is operated fluorescence is produced on a barium platinocyanide screen placed some distance from the tube.
And this effect was traced to rays coming from the walls of the cathode ray tube. Roentgen observed that the rays were very penetrating, being able to traverse several thicknesses of matter. Also many substances which were opaque to ordinary light were transparent to the rays which he subsequently called X-rays according to him for the sake of conciseness.
X-rays are produced whenever high speed electrons are suddenly stopped. This is because the classical electromagnetic theory, whenever charged particles are rapidly accelerated or decelerated, electromagnetic radiation is produced. In details, if the deceleration entails the loss of considerable electron energy within a very short time, the emitted radiation is of very short wavelength.
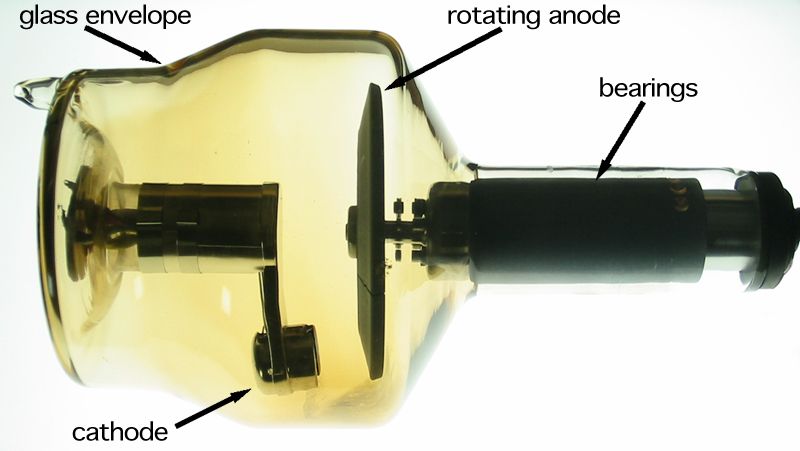
This process can be perceived as an electron produces radiation. Moreover X-rays were first identified when cathode rays were stopped by the glass of the cathode ray tube, they are more efficiently produced when targets of high atomic number and high melting point are used. Resources like Molybdenum, Tungsten and platinum are highly favoured.
TWO PARAMETERS OF RELEVANCE IN THE OPERATION OF AN X-RAY TUBE
The quantity of the emitted X-rays and depends only on the number of electrons incident on the target. This can be controlled by the filament current and this process is called INTENSITY OF THE X-RAY
Penetrating power of the X-rays. This is related to the energy of the emitted X-rays. It depends partly on the nature of the target, but mainly on the accelerating potential. Soft X-rays have small penetrating power and are easily absorbed, while hard X-rays have relatively high penetrating power.
.jpg)
Wikimedia commonsrotating anode x-ray tube
USES AND PROPERTIES OF X-RAYS
The discovery of X-rays revealed that apart from the luminescence they produced on barium platinocyanide and zinc sulphide screens,they also affected photographic plate. X-ray travelled in straight lines from source and are not deflected by electric or magnetic fields, that's why they are not charged particles.
They produce photoelectric effect on certain metals, and like electrons, produce lesser X-rays from metals. Also like electrons they ionize gases through which they pass, and this property has been used in the determination of X-ray intensity. X-rays are electromagnetic waves, having the same nature as light waves. They differ only in wave length or frequency, with the X-rays having the shorter wavelength ranging between 0.1 and 100A.
X-rays penetrate stuff to an extent governed by the density of the object. That's more reason why it is being use in some diagnostic work in medicine. If a hand is placed between the tube and a sensitive screen, the dark shadow of the bones is visible within the slightly Dark Shadow of the hand. The contrast results from the bone being denser than the flesh section of the hand.
This principle is used successfully in diagnosing broken bones and location of the site, also unusual objects such as swallowed safety pins, if dense enough, can be located. Even in materials science, cracks and flaws in metal castings can be discovered from X-rays photo graphs.
X-rays can also be used for therapeutic purposes. It is known that rapidly dividing cells are more readily damaged than stable ones. A carefully controlled dose of X-rays can be used to destroy a growing tumour. Similar techniques are employed with radiations from radioactive isotopes
To observe the stomach and intestinal track, the patient is given a barium meal.This consists of a paste of barium sulpahte and water. Each part of the alimentary canal in turn shows up as the barium meal reaches it. With this any abnormalities and location of such can be detected.
REFERENCES
- The properties of X-ray
- The properties of X-ray and atomic spectra
- The production of X-ray
- Wikipedia: X-ray