A Quest for the Faster Charging Batteries
The average number of hours that an average phone user spends on the phone is approximately 4 hours. That is approximately 120 hours or 5 days. The average daily charging time from 0% to 100% is about 2 hours that makes the number of hours spent charging phone per month is 60 hours or 2 1/2 days. That is about a month in a year. That number is high, and phone manufacturers are continually working on ways to make batteries charge faster with the introduction of Quick Charge technology.

[image credits: Flickr Commons]
This quick Charge was a technology pioneered by Qualcomm Snapdragon, a system on chip (SoC) manufacturers for various devices such as Android and Windows phone. Over time, other SoC manufacturers have come up with their proprietary fast charging technology.
How to charge a battery fast
Battery discharges when ions move from anode to cathode. Reversing the process requires passing current through the electrolyte to push the ions back to the anode. Batteries in small electronic devices such as phones, tablets and laptops usually have their rating in mAh or milliampere hour as against Ah in the deep cycle, golf cart, motor, motorcycle batteries, etc. The bigger a battery is, the longer the charging time. For instance, under same charging condition, a 2,000 mAh battery should charge twice faster than a 4,000 mAh batteries.
Also how fast you charge a battery is a function of both the charging voltage and charging current.
The standard USB charger is 5V at either 0.5A or 1A charging current. If a battery charger outputs 1A 5V, i.e. a 5w charger (power= current x voltage= 5 x 1= 5w), and another charger outputs 2A at same 5V, the latter will charge faster than the former.
Why can't we have a 20A 20V ( or 400w) or more mobile device charger and get the charge over in seconds?
The problem is the present lithium battery technology cannot handle such large amount of charging current. But the Quick Charge technology can cut down the time that it takes other conventional chargers to a quarter of the time- 30 minutes.
The Qualcomm 4.0 version can charge batteries up to a power of 28w. It does this by using a special chip to adjust the maximum current the battery can take plus top of the market heat dissipation technology to avoid battery's temperature from getting to a dangerous level while charging.
The Oliver Twist Effect
Even though with the present fast charging technology we can charge full or at least get to 50% of the mobile phone's capacity, users still complain on 61-watt charger's performance from Apple's iPhone which is commensurate with the steep cost of $69.
Can we have a phone that charge in seconds?
Yes, we can. But sadly the energy source won't be a battery but rather a supercapacitor.
The capacitor and battery have one thing in common - which is an ability to store charge. But all use a different method of storage; a battery makes use of chemical reactions between electrolyte and electrode to cause an electron to flow. Capacitors use electrostatic, the static electricity which occurs when the two plates, separated by a dielectric (an insulating material), takes on charge when an electric current passes through it. A thundercloud is a natural phenomenon that simulates the massive power storing potential of capacitors if the plates get increased to a substantial level.
The Supercapacitors
A supercapacitor is similar to a capacitor but with some distinguishing features which include a higher capacitance and hence can store more energy than ordinary capacitors, a different dielectric material and setup, plus high energy density.

[image credits: Flickr Commons]
Due to the slightly larger plates in the supercapacitor and the decreased separation between the plates, the capacitance of supercapacitors is bigger and can store more charge.
The farad (F) which is the basic unit of measuring capacitance of a capacitor is typically in μF, microfarad (microfarad, one millionth (10−6) of a farad) or picofarad, pF ( (picofarad, one trillionth (10−12) of a farad)) or nF (nanofarad, one billionth (10−9) of a farad) for a capacitor. In a supercapacitor, this ability to store charge is in Farads (F) which means it can store up to a million times more than those in conventional capacitors rated in μF.
Though some firms can pack thousands of farads in a supercapacitor, that amount is still small (around 20%) of what we can do with batteries.
How about portable devices like cell phones that require less power?
A supercapacitor has a lot of good associated with it; one is it can be recharged and discharged for more than 30,000 times without getting weak. That means we are looking at a lifetime of more than ten years. It can, more importantly, charge in seconds. But the drawback is the charge-discharge characteristics and the way the voltage depletes in both batteries and supercapacitors.
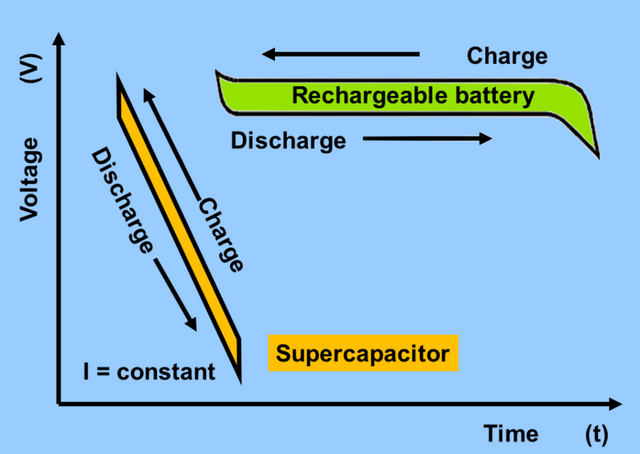
[image credits: Wikipedia Commons]
Look at the graph of voltage against time you will notice the difference between the behaviour of a supercapacitor and a conventional rechargeable battery. The supercapacitor charges as fast as it discharges with its voltage dipping with it. In most electronics, a steady voltage is what is required, for instance, most cellphone's lithium battery voltage is between 3.7v to 4.2v. This battery voltage remains more or less the same until about 90% or more discharged when it dips to cut off voltage of between 3.4v or 3.0v on which battery is assumed to be dead.
For us to use a supercapacitor, there will be more complexity for the added circuitry that will regulate this free fall of voltage.
Also developing a superconductor small enough to fit in a cellphone and with enough capacity as the conventional battery is still a design problem.
Though Eesha Khare, the then high school student of Lynbrook High School in California won an Intel's Young Scientist Award with cash prize of $50,000 in 2013 for developing a supercapacitor that has up to 10,000 cycles and scalable to fit in a phone, and is rechargeable under 20 to 30 seconds. Her demonstration shows the supercapacitor light an LED.
Five years on, the invention may well be on hold. But a new UK-based company called Zap&Go founded in 2013 may well be on the path to be the people to give us the fast charge we may need. They were able to use a nanocarbon-graphene supercapacitor which can charge under 5 minutes which is still a far cry from the 20 seconds charge of the supercapacitors. You can watch the CEO and investment director of Zap&Go talk about the exciting prospect of 5-minute full charge battery here.
But until we have these products available in the open markets, we may have to be content with the 30-minute 50% charge currently offered by the Quick Charge systems :)
REFERENCES
- Qualcomm Quick Charge
- What exactly is Fast Charging? And how does it work?
- We Tested iPhone Fast-Charging and You Should Definitely Upgrade Your Charger
- How Much Time Do People Spend on Their Mobile Phones in 2017?
- Zap&Go
- High school student develops supercapacitor, wins Young Scientist Award
- Supercapacitors
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on discord here. If from Nigeria, there may be need to include the #stemng tag in your post. You can visit this blog by @stemng for more details. You can also check this blog post by @steemstem here and this guidelines here for help on how to be a member of @steemstem. Please also check this blog post from @steemstem on proper use of images devoid of copyright issues here
Would you like to delegate to the @steemstem? Here is a link below
50 SP | 100SP | 500SP | 1,000SP | 5,000SP | 10,000SP | 50,000SP

One thing I have obvserved over the years using mobile phones is that, those ones that charges very fast also tend to discharge at a very high rate.
Recently most mobile phone producers now make phones with inbuilt batteries with high quality and durability, and I have also observed that there is actually a difference. Those phones produced with inbuilt batteries last longer than expected and they don't really charge so fast at times.
I have a Samsung Galaxy that charges within 2hour but doesn't last even up 1 hour
My question :
Does it mean there is a direct relationship between the rate at which a battery discharges and the rate at which it is charged.
You would agree with me that, it is not how heavy a battery is that makes it last longer but rather the cell.
There is no relationship between the way a battery charges and the way it discharges for different makes of batteries. Though there is an exception in weak cells- they tend to charge faster than good batteries of the same make. What determines how long-lasting a battery can be is the battery makeup, i.e. the chemical composition of the electrolyte, electrode, and the type of reaction that produce the electrons.
The weight of a battery also has no direct correlation in performance in different battery type. For instance, lithium-ion batteries weigh much less than lead-acid batteries, but lithium-ion batteries still outperform lead acid ones.
I recently did a health and safety presentation on batteries. Their energy densities are starting to get pretty high and sometimes they can catch fire even while in your pants pockets.
The new developments to make them even more powerful might not be a good thing if you think about it.
Yes, I think we should move from making more powerful batteries to making batteries we can charge in seconds or few minutes.
Nice one @greenrun. I had the quick charge technology in mind when I saw the title. Something went wrong with the quick charge of the Samsung galaxy note 7 which had people returning their phones to Samsung and Samsung eventually stopped the production of the note 7.
With this your explanation I would guess that the problem came with the power tweak they gave Qualcomm snapdragon 845 processor which the phone came with but one funny thing was that they could not figure out where the power issues came from.
Really had fun reading through👍
That Samsung disaster was an error from the design team. Either the batteries charged too fast thereby heating it up to a dangerous level that resulted in explosion or the battery has an internal failure that results in explosion during charge. Samsung is no stranger to exploding batteries. I use Samsung products but never their mobile phones.
That sounds very personal 😅 but Samsung phone's has been one of the best for me. Your argument about the battery hardware I'd no far from the truth but like you said in the post, there might still be issue with the quick charge chip since they were still testing out the technology. Also to prevent the heating issue you pointed out which could also be part of the cause, they implemented a liquid cooling system for their s9 flagship.
Yeah, that's just preference. Let's try other phone makes :)
Very informative post @greenrun
Energy density is still a very big deal when it comes to things like this and it's nice to know that supercapacitors can proffer a solution.
That is true. We need another way of achieving the same goal. It is great to have a fast charging battery if we can't have batteries that last all week long.
Well written @greenrun.
From start to finish was laced with something new to learn.
From Qualcomm fast charging technology which i didnt even know existed.
You also answered a question that had me bothered on why we cant have highly rated chargers to charge our batteries in shorter time.
And the super capacitors.... Cool, but i think we still have far to go in compensating for its downsides.
Its always enlighning on your blog
Oh, I know of the Qualcomm fast charging. It is one of the reason I prefer mobile phones with Qualcomm SoC. The ability of some of the phone with such feature makes for a faster charging.
Hello @greenrun
Very well written to the extent that even a layman will understand what the theme of this piece. That makes you a born tutor!
I guess if the type that can charge under 20seconds or even 5minutes is finally commercialized, it will be an immediate success and the company will make lot of money before completing interests get serious. What a virgin opportunity!
Regards
@eurogee of @euronation and @steemstem communities
Yes, it will help reduce the long hours wasted during charging of portable devices.
Electrolytes are acids that react with anodes and cathodes, causing the cathode to lose the electrons and the anode to get the electrons. As a result, a large number of electrons are built up in the anode and a large number of electrons are removed from the cathode.
We know that an atom or object that has more electrons than a proton is said to be negatively charged. Anode has more electrons than protons. So the anode is said to be a negatively charged electrode. On the other hand, the cathode has a lower number of electrons than protons. So the cathode is said to be a positively charged electrode.
We know that electrons always try to move from higher concentrations (excess electron regions) to lower concentrations (fewer electrons). This is because they are equally distributed on both sides.
Anode is a region of higher electron concentration and the cathode is a lower electron concentration region. So electrons always try to move from anode to cathode. But the electrolyte that exists between the anode and the cathode stops this electron current. As a result, the electrons in the anode can not enter the cathode.
Electrons have no other electrical path to flow from the anode to the cathode. When we make this electrical path or path by connecting the anode and cathode to the external conductor wire, the electrons begin to flow from the anode to the cathode through the wire. In other words, the electric current begins to flow from the anode to the cathode.
When this conductor wire is connected to an electric light bulb, the electrons flowing from the anode through the conductor wire will turn on the electric light bulb, and flow to the cathode. Likewise, we can turn on the device connected to the battery.
Thank you.
Somehow I found this here:
http://www.physics-and-radio-electronics.com/blog/battery-battery-works/
Good comments are worth it, but they also need to be original..
Well written @greenrun, while reading this post, I was happy that finally there is the possibility of charging my phone in seconds but looking at the graph that reminded me of common source and propagated epidemic, I think I would prefer a battery that would store charge and last for a resonable time to supercapacitor that acts like a common source epidemic and discharges rapidly.
I am sorry for bringing biology into this post.
Thank you for this post @greenrun.
It looks like we are stuck in making batteries that last that long as devices are getting "hungrier" by the day. I think if we can charge the batteries faster, that can compensate for the low backup hours of the present battery technology.
Since the world is running faster than usual, I wouldn't mind having the supercapicitor now. Nicely written @greenrun
Thank you.
Nice work @greenrun... I learnt something today
Thank you.
You welcome Bro.
I saw ur upvote, thanks